Long-term efficacy and safety of phrenic nerve stimulation for the treatment of central sleep apnea
- PMID: 31634407
- PMCID: PMC6802564
- DOI: 10.1093/sleep/zsz158
Long-term efficacy and safety of phrenic nerve stimulation for the treatment of central sleep apnea
Abstract
Study objective: To evaluate long-term efficacy and safety of phrenic nerve stimulation (PNS) in patients with moderate-to-severe central sleep apnea (CSA) through 3 years of therapy.
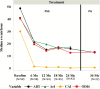
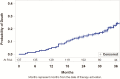
Methods: Patients in the remedē System Pivotal Trial were observed every 3 months after implant until US Food and Drug Administration approval. At the time of approval and study closure, all patients completed 24 months of follow-up; 33 patients had not reached the 36-month visit. Sleep metrics (polysomnography) and echocardiographic parameters are reported at baseline, 12, 18, and 24 months, in addition to available 36-month sleep results from polygraphy. Safety was assessed through 36 months; however, analysis focused through 24 months and available 36-month results are provided.
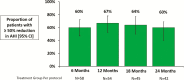
Results: Patients were assessed at 24 (n = 109) and 36 (n = 60) months. Baseline characteristics included mean age 64 years, 91% male, and mean apnea-hypopnea index 47 events per hour. Sleep metrics (apnea-hypopnea index (AHI), central apnea index, arousal index, oxygen desaturation index, rapid eye movement sleep) remained improved through 24 and 36 months with continuous use of PNS therapy. At least 60% of patients in the treatment group achieved at least 50% reduction in AHI through 24 months. Serious adverse events (SAEs) related to the remedē System implant procedure, device, or therapy through 24 months were reported by 10% of patients, no unanticipated adverse device effects or deaths, and all events resolved. No additional related SAEs were reported between 24 and 36 months.
Conclusion: These data suggest beneficial effects of long-term PNS in patients with CSA appear to sustain through 36 months with no new safety concerns.
Trial registration: NCT01816776.
Keywords: central sleep apnea; phrenic nerve stimulation; transvenous stimulation.
© Sleep Research Society 2019. Published by Oxford University Press [on behalf of the Sleep Research Society].
Figures

References
-
- Linz D, et al. . The importance of sleep-disordered breathing in cardiovascular disease. Clin Res Cardiol. 2015;104(9):705–718. - PubMed
-
- Fox H, et al. . Prevalence of sleep-disordered breathing and patient characteristics in a coronary artery disease cohort undergoing cardiovascular rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(6):421–429. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

