2019 update of the drug resistance mutations in HIV-1
- PMID: 31634862
- PMCID: PMC6892618
2019 update of the drug resistance mutations in HIV-1
Abstract
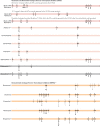
The 2019 edition of the IAS-USA drug resistance mutations list updates the Figure last published in January 2017. The mutations listed are those that have been identified by specific criteria for evidence and drugs described. The Figure is designed to assist practitioners in identifying key mutations associated with resistance to antiretroviral drugs, and therefore, in making clinical decisions regarding antiretroviral therapy.
Figures
References
-
- Gilead Sciences Inc. BIKTARVY [prescribing information]. 2018. Foster City, CA, Gilead Sciences, Inc.
-
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101): 1499–1510. - PubMed
-
- Merck & Co Inc. Doravirine [prescribing information]. 2018. Whitehouse Station, NJ, Merck.
-
- Lam E, Parkin NT. Amprenavir resistance imparted by the I50V mutation in HIV-1 protease can be suppressed by the N88S mutation. Clin Infect Dis. 2003; 37(9):1273–1274. - PubMed
References to the User Notes
-
- von Wyl V, Ehteshami M, Demeter LM, et al. HIV-1 reverse transcriptase connection domain mutations: dynamics of emergence and implications for success of combination antiretroviral therapy. Clin Infect Dis. 2010; 51(5):620–628. - PubMed
-
- Rimsky L, Van Eygen V, Vingerhoets J, Leijskens E, Picchio G. Reverse transcriptase connection domain mutations were not associated with virological failure or phenotypic resistance in rilpivirine-treated patients from the ECHO and THRIVE Phase III trials (week 96 analysis). Antivir Ther. 2012;17(Suppl 1):A36.
-
- Miller MD, Margot N, Lu B, et al. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004;189(5):837–846. - PubMed
-
- Harrigan PR, Stone C, Griffin P, et al. Resistance profile of the human immunodeficiency virus type 1 reverse transcriptase inhibitor abacavir (1592U89) after monotherapy and combination therapy. CNA2001 Investigative Group. J Infect Dis. 2000;181(3):912–920. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical