Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway
- PMID: 31635020
- PMCID: PMC6843682
- DOI: 10.3390/microorganisms7100472
Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway
Abstract
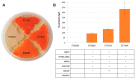
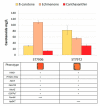
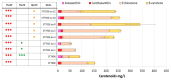
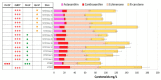
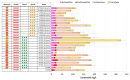
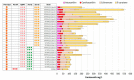
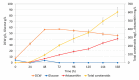
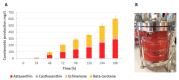
Astaxanthin is a high-value red pigment and antioxidant used by pharmaceutical, cosmetics, and food industries. The astaxanthin produced chemically is costly and is not approved for human consumption due to the presence of by-products. The astaxanthin production by natural microalgae requires large open areas and specialized equipment, the process takes a long time, and results in low titers. Recombinant microbial cell factories can be engineered to produce astaxanthin by fermentation in standard equipment. In this work, an oleaginous yeast Yarrowia lipolytica was engineered to produce astaxanthin at high titers in submerged fermentation. First, a platform strain was created with an optimised pathway towards β-carotene. The platform strain produced 331 ± 66 mg/L of β-carotene in small-scale cultivation, with the cellular content of 2.25% of dry cell weight. Next, the genes encoding β-ketolase and β-hydroxylase of bacterial (Paracoccus sp. and Pantoea ananatis) and algal (Haematococcus pluvialis) origins were introduced into the platform strain in different copy numbers. The resulting strains were screened for astaxanthin production, and the best strain, containing algal β-ketolase and β-hydroxylase, resulted in astaxanthin titer of 44 ± 1 mg/L. The same strain was cultivated in controlled bioreactors, and a titer of 285 ± 19 mg/L of astaxanthin was obtained after seven days of fermentation on complex medium with glucose. Our study shows the potential of Y. lipolytica as the cell factory for astaxanthin production.
Keywords: Yarrowia lipolytica; astaxanthin; metabolic engineering; submerged fermentation; β-carotene.
Conflict of interest statement
I.B. and K.R.K. have financial interest in BioPhero ApS.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

