Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells
- PMID: 31635074
- PMCID: PMC6836113
- DOI: 10.3390/nu11102525
Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells
Abstract
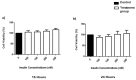
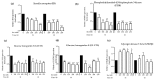
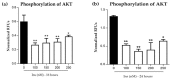
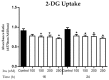
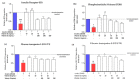
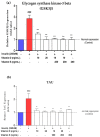
This study investigated the effects of vitamins D and E on an insulin-resistant model and hypothesized that this treatment would reverse the effects of Alzheimer's disease (AD) and improves insulin signalling. An insulin-resistant model was induced in SK-N-SH neuronal cells with a treatment of 250 nM insulin and re-challenged with 100 nM at two different incubation time (16 h and 24 h). The effects of vitamin D (10 and 20 ng/mL), vitamin E in the form of tocotrienol-rich fraction (TRF) (200 ng/mL) and the combination of vitamins D and E on insulin signalling markers (IR, PI3K, GLUT3, GLUT4, and p-AKT), glucose uptake and AD markers (GSK3β and TAU) were determined using quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA). The results demonstrated an improvement of the insulin signalling pathway upon treatment with vitamin D alone, with significant increases in IR, PI3K, GLUT3, GLUT4 expression levels, as well as AKT phosphorylation and glucose uptake, while GSK3β and TAU expression levels was decreased significantly. On the contrary, vitamin E alone, increased p-AKT, reduced the ROS as well as GSK3β and TAU but had no effect on the insulin signalling expression levels. The combination of vitamins D and E only showed significant increase in GLUT4, p-AKT, reduced ROS as well as GSK3β and TAU. Thus, the universal role of vitamin D, E alone and in combinations could be the potential nutritional agents in restoring the sensitivity of neuronal cells towards insulin and delaying the pathophysiological progression of AD.
Keywords: SK-N-SH neuronal cells; glucose uptake; insulin resistance; vitamin D; vitamin E.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Talbot K., Wang H.-Y., Kazi H., Han L.-Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., et al. Demonstrated Brain Insulin Resistance in Alzheimer’s Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Investig. 2012;122:1316–1338. doi: 10.1172/JCI59903. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

