Atherosclerosis and Coenzyme Q10
- PMID: 31635164
- PMCID: PMC6834161
- DOI: 10.3390/ijms20205195
Atherosclerosis and Coenzyme Q10
Abstract
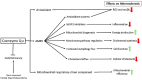
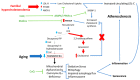
Atherosclerosis is the most common cause of cardiac deaths worldwide. Classically, atherosclerosis has been explained as a simple arterial lipid deposition with concomitant loss of vascular elasticity. Eventually, this condition can lead to consequent blood flow reduction through the affected vessel. However, numerous studies have demonstrated that more factors than lipid accumulation are involved in arterial damage at the cellular level, such as inflammation, autophagy impairment, mitochondrial dysfunction, and/or free-radical overproduction. In order to consider the correction of all of these pathological changes, new approaches in atherosclerosis treatment are necessary. Ubiquinone or coenzyme Q10 is a multifunctional molecule that could theoretically revert most of the cellular alterations found in atherosclerosis, such as cholesterol biosynthesis dysregulation, impaired autophagy flux and mitochondrial dysfunction thanks to its redox and signaling properties. In this review, we will show the latest advances in the knowledge of the relationships between coenzyme Q10 and atherosclerosis. In addition, as atherosclerosis phenotype is closely related to aging, it is reasonable to believe that coenzyme Q10 supplementation could be beneficial for both conditions.
Keywords: aging; atherosclerosis; coenzyme Q10; ubiquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. - DOI - PMC - PubMed

