Influence of Hydrogen Electron Donor, Alkaline pH, and High Nitrate Concentrations on Microbial Denitrification: A Review
- PMID: 31635215
- PMCID: PMC6834205
- DOI: 10.3390/ijms20205163
Influence of Hydrogen Electron Donor, Alkaline pH, and High Nitrate Concentrations on Microbial Denitrification: A Review
Abstract
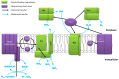
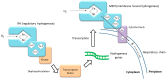
Bacterial respiration of nitrate is a natural process of nitrate reduction, which has been industrialized to treat anthropic nitrate pollution. This process, also known as "microbial denitrification", is widely documented from the fundamental and engineering points of view for the enhancement of the removal of nitrate in wastewater. For this purpose, experiments are generally conducted with heterotrophic microbial metabolism, neutral pH and moderate nitrate concentrations (<50 mM). The present review focuses on a different approach as it aims to understand the effects of hydrogenotrophy, alkaline pH and high nitrate concentration on microbial denitrification. Hydrogen has a high energy content but its low solubility, 0.74 mM (1 atm, 30 °C), in aqueous medium limits its bioavailability, putting it at a kinetic disadvantage compared to more soluble organic compounds. For most bacteria, the optimal pH varies between 7.5 and 9.5. Outside this range, denitrification is slowed down and nitrite (NO2-) accumulates. Some alkaliphilic bacteria are able to express denitrifying activity at pH levels close to 12 thanks to specific adaptation and resistance mechanisms detailed in this manuscript, and some bacterial populations support nitrate concentrations in the range of several hundred mM to 1 M. A high concentration of nitrate generally leads to an accumulation of nitrite. Nitrite accumulation can inhibit bacterial activity and may be a cause of cell death.
Keywords: acclimation; denitrifying bacteria; high nitrate concentration; high pH; hydrogenotrophic denitrification; mineral carbon; nitrite accumulation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Mohsenipour M., Shahid S., Ebrahimi K. Removal techniques of nitrate from water. Asian J. Chem. 2014;26:7881–7886. doi: 10.14233/ajchem.2014.17136. - DOI
-
- Kapoor A., Viraraghavan T. Nitrate Removal from Drinking Water—Review. J. Environ. Eng. 1997;123:371–380. doi: 10.1061/(ASCE)0733-9372(1997)123:4(371). - DOI
-
- Francis C.W., Hatcher C.W. Biological Denitrification of High-Nitrates Wastes Generated in the Nuclear Industry. Environmental Sciences Division, Oak Ridge National Laboratory; Oak Ridge, TN, USA: 1980.
-
- Albrecht A., Bertron A., Libert M. Cement-Based Materials for Nuclear Waste Storage. Springer; New York, NY, USA: 2013. Microbial Catalysis of Redox Reactions in Concrete Cells of Nuclear Waste Repositories: A Review and Introduction; pp. 147–159.
-
- Stroes-Gascoyne S., Sergeant C., Schippers A., Hamon C.J., Nèble S., Vesvres M.-H., Barsotti V., Poulain S., Le Marrec C. Biogeochemical processes in a clay formation in situ experiment: Part D—Microbial analyses—Synthesis of results. Appl. Geochem. 2011;26:980–989. doi: 10.1016/j.apgeochem.2011.03.007. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

