Repurposing the aldose reductase inhibitor and diabetic neuropathy drug epalrestat for the congenital disorder of glycosylation PMM2-CDG
- PMID: 31636082
- PMCID: PMC6899038
- DOI: 10.1242/dmm.040584
Repurposing the aldose reductase inhibitor and diabetic neuropathy drug epalrestat for the congenital disorder of glycosylation PMM2-CDG
Abstract
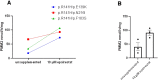
Phosphomannomutase 2 deficiency, or PMM2-CDG, is the most common congenital disorder of glycosylation and affects over 1000 patients globally. There are no approved drugs that treat the symptoms or root cause of PMM2-CDG. To identify clinically actionable compounds that boost human PMM2 enzyme function, we performed a multispecies drug repurposing screen using a novel worm model of PMM2-CDG, followed by PMM2 enzyme functional studies in PMM2-CDG patient fibroblasts. Drug repurposing candidates from this study, and drug repurposing candidates from a previously published study using yeast models of PMM2-CDG, were tested for their effect on human PMM2 enzyme activity in PMM2-CDG fibroblasts. Of the 20 repurposing candidates discovered in the worm-based phenotypic screen, 12 were plant-based polyphenols. Insights from structure-activity relationships revealed epalrestat, the only antidiabetic aldose reductase inhibitor approved for use in humans, as a first-in-class PMM2 enzyme activator. Epalrestat increased PMM2 enzymatic activity in four PMM2-CDG patient fibroblast lines with genotypes R141H/F119L, R141H/E139K, R141H/N216I and R141H/F183S. PMM2 enzyme activity gains ranged from 30% to 400% over baseline, depending on genotype. Pharmacological inhibition of aldose reductase by epalrestat may shunt glucose from the polyol pathway to glucose-1,6-bisphosphate, which is an endogenous stabilizer and coactivator of PMM2 homodimerization. Epalrestat is a safe, oral and brain penetrant drug that was approved 27 years ago in Japan to treat diabetic neuropathy in geriatric populations. We demonstrate that epalrestat is the first small molecule activator of PMM2 enzyme activity with the potential to treat peripheral neuropathy and correct the underlying enzyme deficiency in a majority of pediatric and adult PMM2-CDG patients.
Keywords: Aldose reductase inhibitor; Congenital disorder of glycosylation; Drug repurposing; Epalrestat; PMM2-CDG; Phosphomannomutase 2 deficiency.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe following authors are shareholders of Perlara PBC: S.I., N.D., F.S.S., Z.P., K.M., H.T. and E.O.P.
Figures





References
-
- Cabezas O. R., Flanagan S. E., Stanescu H., García-Martínez E., Caswell R., Lango-Allen H., Antón-Gamero M., Argente J., Bussell A.-M., Brandli A. et al. (2017). Polycystic kidney disease with hyperinsulinemic hypoglycemia caused by a promoter mutation in phosphomannomutase 2. J. Am. Soc. Nephrol. 28, 2529-2539. 10.1681/ASN.2016121312 - DOI - PMC - PubMed
-
- Citro V., Cimmaruta C., Monticelli M., Riccio G., Hay Mele B., Cubellis M. V. and Andreotti G. (2018). The analysis of variants in the general population reveals that PMM2 is extremely tolerant to missense mutations and that diagnosis of PMM2-CDG can benefit from the identification of modifiers. Int. J. Mol. Sci. 19, 2218 10.3390/ijms19082218 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

