Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention
- PMID: 31636087
- PMCID: PMC7057308
- DOI: 10.2215/CJN.07420619
Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention
Abstract
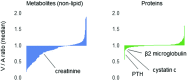
In this review of the application of proteomics and metabolomics to kidney disease research, we review key concepts, highlight illustrative examples, and outline future directions. The proteome and metabolome reflect the influence of environmental exposures in addition to genetic coding. Circulating levels of proteins and metabolites are dynamic and modifiable, and thus amenable to therapeutic targeting. Design and analytic considerations in proteomics and metabolomics studies should be tailored to the investigator's goals. For the identification of clinical biomarkers, adjustment for all potential confounding variables, particularly GFR, and strict significance thresholds are warranted. However, this approach has the potential to obscure biologic signals and can be overly conservative given the high degree of intercorrelation within the proteome and metabolome. Mass spectrometry, often coupled to up-front chromatographic separation techniques, is a major workhorse in both proteomics and metabolomics. High-throughput antibody- and aptamer-based proteomic platforms have emerged as additional, powerful approaches to assay the proteome. As the breadth of coverage for these methodologies continues to expand, machine learning tools and pathway analyses can help select the molecules of greatest interest and categorize them in distinct biologic themes. Studies to date have already made a substantial effect, for example elucidating target antigens in membranous nephropathy, identifying a signature of urinary peptides that adds prognostic information to urinary albumin in CKD, implicating circulating inflammatory proteins as potential mediators of diabetic nephropathy, demonstrating the key role of the microbiome in the uremic milieu, and highlighting kidney bioenergetics as a modifiable factor in AKI. Additional studies are required to replicate and expand on these findings in independent cohorts. Further, more work is needed to understand the longitudinal trajectory of select protein and metabolite markers, perform transomics analyses within merged datasets, and incorporate more kidney tissue-based investigation.
Keywords: Kidney Genomics Series; Metabolomics; albumins; biological products; biomarkers; chronic renal insufficiency; confounding factors (epidemiology); diabetic nephropathies; diabetic nephropathy; energy metabolism; environmental exposure; goals; kidney; machine learning; mass spectrometry; membranous glomerulonephritis; metabolome; microbiota; peptides; prognosis; proteome; proteomics; research personnel.
Copyright © 2020 by the American Society of Nephrology.
Figures

References
-
- Sauer S, Lange BM, Gobom J, Nyarsik L, Seitz H, Lehrach H: Miniaturization in functional genomics and proteomics. Nat Rev Genet 6: 465–476, 2005 - PubMed
-
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F: Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015 - PubMed
-
- Hoyer KJR, Dittrich S, Bartram MP, Rinschen MM: Quantification of molecular heterogeneity in kidney tissue by targeted proteomics. J Proteomics 193: 85–92, 2019 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

