Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies
- PMID: 31636208
- PMCID: PMC6842624
- DOI: 10.1073/pnas.1821218116
Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies
Abstract
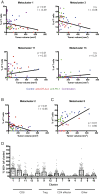
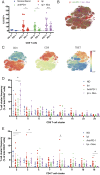
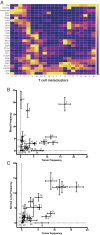
Immune checkpoint blockade therapy targets T cell-negative costimulatory molecules such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1). Combination anti-CTLA-4 and anti-PD-1 blockade therapy has enhanced efficacy, but it remains unclear through what mechanisms such effects are mediated. A critical question is whether combination therapy targets and modulates the same T cell populations as monotherapies. Using a mass cytometry-based systems approach, we comprehensively profiled the response of T cell populations to monotherapy and combination anti-CTLA-4 plus anti-PD-1 therapy in syngeneic murine tumors and clinical samples. Most effects of monotherapies were additive in the context of combination therapy; however, multiple combination therapy-specific effects were observed. Highly phenotypically exhausted cluster of differentiation 8 (CD8) T cells expand in frequency following anti-PD-1 monotherapy but not combination therapy, while activated terminally differentiated effector CD8 T cells expand only following combination therapy. Combination therapy also led to further increased frequency of T helper type 1 (Th1)-like CD4 effector T cells even though anti-PD-1 monotherapy is not sufficient to do so. Mass cytometry analyses of peripheral blood from melanoma patients treated with immune checkpoint blockade therapies similarly revealed mostly additive effects on the frequencies of T cell subsets along with unique modulation of terminally differentiated effector CD8 T cells by combination ipilimumab plus nivolumab therapy. Together, these findings indicate that dual blockade of CTLA-4 and PD-1 therapy is sufficient to induce unique cellular responses compared with either monotherapy.
Keywords: CTLA-4; PD-1; T cell; immune checkpoint blockade; mass cytometry.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: S.C.W. is currently an employee of Spotlight Therapeutics. J.P.A. is a cofounder of Jounce and Neon Therapeutics. J.P.A. has ownership interest in Jounce Therapeutics, Neon Therapeutics, Forty Seven, ImaginAb, Marker Therapeutics, Tvardi, Constellation, BioAtla, Polaris, and Apricity; is a scientific advisory board member/consultant for Jounce, BioAtla, Neon, Amgen, Forty Seven, ImaginAb, Marker Therapeutics, Apricity, Polaris, Oncolytics, and Pieris; and has received royalties from intellectual property licensed to BMS and Merck. M.C.A. reports travel support and honoraria from Merck unrelated to the current work. J.A.W. is a paid speaker for Imedex, Dava Oncology, Omniprex, Illumina, Gilead, MedImmune, and Bristol Meyers Squibb. J.A.W. is a consultant/advisory board member for Roche-Genentech, Novartis, Astra-Zeneca, Glaxo Smith Klein, Bristol Meyers Squibb, Merck, and Microbiome DX. J.A.W. also receives clinical trial support from Glaxo Smith Klein, Roche-Genentech, Bristol Meyers Squibb, and Novartis. J.A.W. is a clinical and scientific advisor at Microbiome DX and a consultant at Biothera Pharma, Merck Sharp, and Dohme. J.A.W. is an inventor on a US patent application submitted by The University of Texas MD Anderson Cancer Center that covers methods to enhance checkpoint blockade therapy by the microbiome. Reviewer R.A. holds patents on programmed cell death-1–targeted cancer therapies.
Figures

References
-
- Sharma P., Allison J. P., The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
-
- Tang J., Shalabi A., Hubbard-Lucey V. M., Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 29, 84–91 (2018). - PubMed
-
- Wei S. C., Duffy C. R., Allison J. P., Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

