Functional characterization of 84 PALB2 variants of uncertain significance
- PMID: 31636395
- PMCID: PMC7056643
- DOI: 10.1038/s41436-019-0682-z
Functional characterization of 84 PALB2 variants of uncertain significance
Abstract
Purpose: Inherited pathogenic variants in PALB2 are associated with increased risk of breast and pancreatic cancer. However, the functional and clinical relevance of many missense variants of uncertain significance (VUS) identified through clinical genetic testing is unclear. The ability of patient-derived germline missense VUS to disrupt PALB2 function was assessed to identify variants with potential clinical relevance.
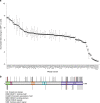
Methods: The influence of 84 VUS on PALB2 function was evaluated using a cellular homology directed DNA repair (HDR) assay and VUS impacting activity were further characterized using secondary functional assays.
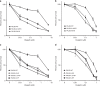
Results: Four (~5%) variants (p.L24S,c.71T>C; p.L35P,c.104T>C; pI944N,c.2831T>A; and p.L1070P,c.3209T>C) disrupted PALB2-mediated HDR activity. These variants conferred sensitivity to cisplatin and a poly(ADP-ribose) polymerase (PARP) inhibitor and reduced RAD51 foci formation in response to DNA damage. The p.L24S and p.L35P variants disrupted BRCA1-PALB2 protein complexes, p.I944N was associated with protein instability, and both p.I944N and p.L1070P mislocalized PALB2 to the cytoplasm.
Conclusion: These findings show that the HDR assay is an effective method for screening the influence of inherited variants on PALB2 function, that four missense variants impact PALB2 function and may influence cancer risk and response to therapy, and suggest that few inherited PALB2 missense variants disrupt PALB2 function in DNA repair.
Keywords: PALB2; PARP inhibitor; breast cancer; homologous recombination repair; variant of uncertain significance (VUS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Comment in
-
PALB2 Genetic Variants: Can Functional Assays Assist Translation?Trends Cancer. 2020 Apr;6(4):263-265. doi: 10.1016/j.trecan.2020.01.017. Epub 2020 Feb 13. Trends Cancer. 2020. PMID: 32209438
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

