Benchmarking of survival outcomes following haematopoietic stem cell transplantation: A review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE)
- PMID: 31636397
- PMCID: PMC7113189
- DOI: 10.1038/s41409-019-0718-7
Benchmarking of survival outcomes following haematopoietic stem cell transplantation: A review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE)
Erratum in
-
Correction: Benchmarking of survival outcomes following haematopoietic stem cell transplantation: A review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE).Bone Marrow Transplant. 2020 Apr;55(4):838-839. doi: 10.1038/s41409-019-0740-9. Bone Marrow Transplant. 2020. PMID: 31754251 Free PMC article.
Abstract
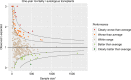
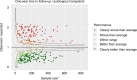
In many healthcare settings, benchmarking for complex procedures has become a mandatory requirement by competent authorities, regulators, payers and patients to assure clinical performance, cost-effectiveness and safe care of patients. In several countries inside and outside Europe, benchmarking systems have been established for haematopoietic stem cell transplantation (HSCT), but access is not universal. As benchmarking is now integrated into the FACT-JACIE standards, the EBMT and JACIE established a Clinical Outcomes Group (COG) to develop and introduce a universal system accessible across EBMT members. Established systems from seven European countries (United Kingdom, Italy, Belgium, France, Germany, Spain, Switzerland), USA and Australia were appraised, revealing similarities in process, but wide variations in selection criteria and statistical methods. In tandem, the COG developed the first phase of a bespoke risk-adapted international benchmarking model for one-year survival following allogeneic and autologous HSCT based on current capabilities within the EBMT registry core dataset. Data completeness, which has a critical impact on validity of centre comparisons, is also assessed. Ongoing development will include further scientific validation of the model, incorporation of further variables (when appropriate) alongside implementation of systems for clinically meaningful interpretation and governance aiming to maximise acceptance to centres, clinicians, payers and patients across EBMT.
Conflict of interest statement
JAS declares speaker fees at educational events supported by Sanofi, Janssen, Jazz, Mallinckrodt and Gilead, and is a member of a trial IDMC for Kiadis Pharma. Other authors declare no conflicts of interest.
Figures



References
-
- Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;84:342–9. doi: 10.1097/TA.0000000000001758. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

