Pan-viral serology implicates enteroviruses in acute flaccid myelitis
- PMID: 31636453
- PMCID: PMC6858576
- DOI: 10.1038/s41591-019-0613-1
Pan-viral serology implicates enteroviruses in acute flaccid myelitis
Erratum in
-
Author Correction: Pan-viral serology implicates enteroviruses in acute flaccid myelitis.Nat Med. 2021 Oct;27(10):1849. doi: 10.1038/s41591-021-01429-z. Nat Med. 2021. PMID: 34548659 No abstract available.
Abstract
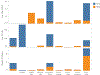
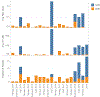
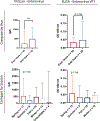
Since 2012, the United States of America has experienced a biennial spike in pediatric acute flaccid myelitis (AFM)1-6. Epidemiologic evidence suggests non-polio enteroviruses (EVs) are a potential etiology, yet EV RNA is rarely detected in cerebrospinal fluid (CSF)2. CSF from children with AFM (n = 42) and other pediatric neurologic disease controls (n = 58) were investigated for intrathecal antiviral antibodies, using a phage display library expressing 481,966 overlapping peptides derived from all known vertebrate and arboviruses (VirScan). Metagenomic next-generation sequencing (mNGS) of AFM CSF RNA (n = 20 cases) was also performed, both unbiased sequencing and with targeted enrichment for EVs. Using VirScan, the viral family significantly enriched by the CSF of AFM cases relative to controls was Picornaviridae, with the most enriched Picornaviridae peptides belonging to the genus Enterovirus (n = 29/42 cases versus 4/58 controls). EV VP1 ELISA confirmed this finding (n = 22/26 cases versus 7/50 controls). mNGS did not detect additional EV RNA. Despite rare detection of EV RNA, pan-viral serology frequently identified high levels of CSF EV-specific antibodies in AFM compared with controls, providing further evidence for a causal role of non-polio EVs in AFM.
Conflict of interest statement
Competing Interests Statement
The authors have no competing interests to declare.
Figures



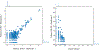





References
-
- Van Haren K, et al. Acute Flaccid Myelitis of Unknown Etiology in California, 2012–2015. JAMA 314, 2663–2671 (2015). - PubMed
-
- CDC AFM Confirmed U.S. Cases To Date. (2019).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

