Therapeutic inhibition of mTORC2 rescues the behavioral and neurophysiological abnormalities associated with Pten-deficiency
- PMID: 31636454
- PMCID: PMC7082835
- DOI: 10.1038/s41591-019-0608-y
Therapeutic inhibition of mTORC2 rescues the behavioral and neurophysiological abnormalities associated with Pten-deficiency
Abstract
Dysregulation of the mammalian target of rapamycin (mTOR) signaling, which is mediated by two structurally and functionally distinct complexes, mTORC1 and mTORC2, has been implicated in several neurological disorders1-3. Individuals carrying loss-of-function mutations in the phosphatase and tensin homolog (PTEN) gene, a negative regulator of mTOR signaling, are prone to developing macrocephaly, autism spectrum disorder (ASD), seizures and intellectual disability2,4,5. It is generally believed that the neurological symptoms associated with loss of PTEN and other mTORopathies (for example, mutations in the tuberous sclerosis genes TSC1 or TSC2) are due to hyperactivation of mTORC1-mediated protein synthesis1,2,4,6,7. Using molecular genetics, we unexpectedly found that genetic deletion of mTORC2 (but not mTORC1) activity prolonged lifespan, suppressed seizures, rescued ASD-like behaviors and long-term memory, and normalized metabolic changes in the brain of mice lacking Pten. In a more therapeutically oriented approach, we found that administration of an antisense oligonucleotide (ASO) targeting mTORC2's defining component Rictor specifically inhibits mTORC2 activity and reverses the behavioral and neurophysiological abnormalities in adolescent Pten-deficient mice. Collectively, our findings indicate that mTORC2 is the major driver underlying the neuropathophysiology associated with Pten-deficiency, and its therapeutic reduction could represent a promising and broadly effective translational therapy for neurological disorders where mTOR signaling is dysregulated.
Figures


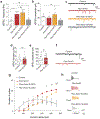






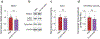




Comment in
-
Therapeutic Targeting of mTORC2 in mTORopathies.Neuron. 2019 Dec 18;104(6):1032-1033. doi: 10.1016/j.neuron.2019.11.026. Neuron. 2019. PMID: 31951535
-
mTORC2 Steals the Spotlight.Epilepsy Curr. 2020 Mar;20(2):116-117. doi: 10.1177/1535759720905835. Epub 2020 Feb 26. Epilepsy Curr. 2020. PMID: 32100554 Free PMC article.
References
-
- Winden KD, Ebrahimi-Fakhari D & Sahin M Abnormal mTOR Activation in Autism. Annual review of neuroscience (2018). - PubMed
-
- Costa-Mattioli M & Monteggia LM mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nature neuroscience 16, 1537–1543 (2013). - PubMed
-
- Zhou J & Parada LF PTEN signaling in autism spectrum disorders. Current opinion in neurobiology (2012). - PubMed
-
- Knafo S & Esteban JA PTEN: Local and Global Modulation of Neuronal Function in Health and Disease. Trends in neurosciences 40, 83–91 (2017). - PubMed
Methods-only References
-
- Kwon CH, et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nature genetics 29, 404–411 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

