Prevalence of hepatocarcinoma-related hepatitis B virus mutants in patients in grey zone of treatment
- PMID: 31636479
- PMCID: PMC6801187
- DOI: 10.3748/wjg.v25.i38.5883
Prevalence of hepatocarcinoma-related hepatitis B virus mutants in patients in grey zone of treatment
Abstract
Background: Antiviral treatment of patients with chronic hepatitis B (CHB) in the grey zone of treatment comands risk management in order to optimize the health outcome. In this sense, the identification of HBV mutants related with an increased risk of hepatocellular carcinoma (HCC) could be useful to identify subpopulations with potential indication of antiviral treatment.
Aim: To analyze the prevalence/persistence of hepatitis B virus (HBV) preS and basal core promoter (BCP)/precore/core variants associated to HCC development in CHB patients in the grey zone.
Methods: Work was designed as a longitudinal retrospective study, including 106 plasma samples from 31 patients with CHB in the grey zone of treatment: Hepatitis B e antigen negative, HBV-DNA levels between 12-20000 IU/mL, normal or discordant transaminase levels during follow up and mild/moderate necro-inflammatory activity in liver biopsy or Fibroscan (up to 9.5 kPa). Serum HBV-DNA was tested using the Abbott Real Time HBV Assay and the BCP/precore/core and the hepatitis B surface antigen (HBsAg) coding regions were analyzed in positive samples by PCR/bulk-sequencing to identify the HCC-related HBV mutants.
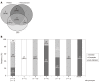
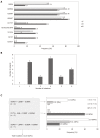
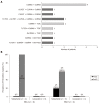
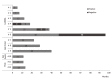
Results: High-risk HCC related mutants were detected in 24 (77%) patients: 19 (61%) in the BCP/precore/core, and 7 (23%) in the HBsAg coding region (2 preS1 and 5 preS2 deletions). The prevalence of preS deletions was genotype-dependent: 3/5 (60%) patients with preS2 deletions and 1/2 with preS1 deletions were infected with the HBV-E genotype. Since HBV-E was the most prevalent in sub-Saharan patients, a correlation between preS deletions and ethnicity was also found: 6/8 (75%) sub-Saharan vs 1/19 (5%) Caucasian patients had preS deletions (P = 0.00016). Remarkably, this correlation was maintained in those patients infected with HBV-A, a minor genotype in sub-Saharan patients: 2/2 patients infected with HBV-A from West Africa vs 0/6 of Caucasian origin had preS deletions. The HCC related variants were the major strains and persisted over time (up to 48 mo). Patients with preS deletions had a significant higher prevalence of F2 fibrosis stage than the negatives (57% vs 10%, P = 0.0078).
Conclusion: HBV genetic analysis of selected populations, like sub-Saharans infected with HBV-E/A genotypes, will allow identification of subpopulations with risk of HCC development due to accumulation of high-risk HBV variants, thus commanding their increased clinical surveillance.
Keywords: Grey zone; Hepatitis B virus; Hepatitis B virus treatment; Hepatocellular carcinoma; PreS deletions.
©The Author(s) 2019. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors have nothing to disclose.
Figures


Similar articles
-
Molecular characterization of hepatitis B virus isolated from Black South African cancer patients, with and without hepatocellular carcinoma.Arch Virol. 2020 Aug;165(8):1815-1825. doi: 10.1007/s00705-020-04686-4. Epub 2020 Jun 5. Arch Virol. 2020. PMID: 32504396
-
Clinical Implications of HBV PreS/S Mutations and the Effects of PreS2 Deletion on Mitochondria, Liver Fibrosis, and Cancer Development.Hepatology. 2021 Aug;74(2):641-655. doi: 10.1002/hep.31789. Epub 2021 May 26. Hepatology. 2021. PMID: 33675094
-
Genomic change in hepatitis B virus associated with development of hepatocellular carcinoma.World J Gastroenterol. 2016 Jun 21;22(23):5393-9. doi: 10.3748/wjg.v22.i23.5393. World J Gastroenterol. 2016. PMID: 27340355 Free PMC article.
-
The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets.J Pathol. 2015 Jan;235(2):355-67. doi: 10.1002/path.4434. J Pathol. 2015. PMID: 25196558 Review.
-
Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J.Semin Liver Dis. 2013 May;33(2):97-102. doi: 10.1055/s-0033-1345716. Epub 2013 Jun 8. Semin Liver Dis. 2013. PMID: 23749665 Review.
Cited by
-
Hepatitis B Virus DNA Integration and Clonal Expansion of Hepatocytes in the Chronically Infected Liver.Viruses. 2021 Jan 30;13(2):210. doi: 10.3390/v13020210. Viruses. 2021. PMID: 33573130 Free PMC article. Review.
-
Hepatitis B virus infection specially increases risk of liver metastasis in breast cancer patients: a propensity-matched analysis.Transl Cancer Res. 2020 Mar;9(3):1506-1517. doi: 10.21037/tcr.2020.01.63. Transl Cancer Res. 2020. PMID: 35117498 Free PMC article.
-
HBV preS Mutations Promote Hepatocarcinogenesis by Inducing Endoplasmic Reticulum Stress and Upregulating Inflammatory Signaling.Cancers (Basel). 2022 Jul 4;14(13):3274. doi: 10.3390/cancers14133274. Cancers (Basel). 2022. PMID: 35805045 Free PMC article.
-
Immune infiltration-associated serum amyloid A1 predicts favorable prognosis for hepatocellular carcinoma.World J Gastroenterol. 2020 Sep 21;26(35):5287-5301. doi: 10.3748/wjg.v26.i35.5287. World J Gastroenterol. 2020. PMID: 32994688 Free PMC article.
-
Whole genome analysis of hepatitis B virus before and during long-term therapy in chronic infected patients: Molecular characterization, impact on treatment and liver disease progression.Front Microbiol. 2022 Oct 17;13:1020147. doi: 10.3389/fmicb.2022.1020147. eCollection 2022. Front Microbiol. 2022. PMID: 36325017 Free PMC article.
References
-
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
-
- Ghany MG. Current treatment guidelines of chronic hepatitis B: The role of nucleos(t)ide analogues and peginterferon. Best Pract Res Clin Gastroenterol. 2017;31:299–309. - PubMed
-
- Marciano S, Gadano A. Why not to stop antiviral treatment in patients with chronic hepatitis B. Liver Int. 2018;38 Suppl 1:97–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

