Trypanosoma cruzi Mexican Strains Differentially Modulate Surface Markers and Cytokine Production in Bone Marrow-Derived Dendritic Cells from C57BL/6 and BALB/c Mice
- PMID: 31636507
- PMCID: PMC6766131
- DOI: 10.1155/2019/7214798
Trypanosoma cruzi Mexican Strains Differentially Modulate Surface Markers and Cytokine Production in Bone Marrow-Derived Dendritic Cells from C57BL/6 and BALB/c Mice
Abstract
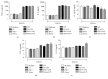
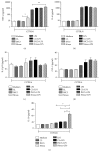
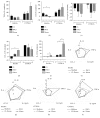
Dendritic cells (DCs) are a type of antigen-presenting cells that play an important role in the immune response against Trypanosoma cruzi, the causative agent of Chagas disease. In vitro and in vivo studies have shown that the modulation of these cells by this parasite can directly affect the innate and acquired immune response of the host in order to facilitate its biological cycle and the spreading of the species. Many studies show the mechanisms by which T. cruzi modulates DCs, but the interaction of these cells with the Mexican strains of T. cruzi such as Ninoa and INC5 has not yet been properly investigated. Here, we evaluated whether Ninoa and INC5 strains evaded the immunity of their hosts by modulating the biology and function of murine DCs. The CL-Brener strain was used as the reference strain. Herein, it was demonstrated that Ninoa was more infective toward bone marrow-derived dendritic cells (BMDCs) than INC5 and CL-Brener strains in both BMDCs of BALB/c and C57BL/6 mice. Mexican strains of T. cruzi induced different cytokine patterns. In BMDCs obtained from BALB/c mice, Ninoa strain led to the reduction in IL-6 and increased IL-10 production, while in C57BL/6 mice Ninoa strain considerably increased the productions of TNF-α and IL-10. Also, Ninoa and INC5 differentially modulated BMDC expressions of MHC-II, TLR2, and TLR4 in both BALB/c and C57BL/6 mice compared to Brazilian strain CL-Brener. These results indicate that T. cruzi Mexican strains differentially infect and modulate MHC-II, toll-like receptors, and cytokine production in DCs obtained from C57BL/6 and BALB/c mice, suggesting that these strains have developed particular modulatory strategies to disrupt DCs and, consequently, the host immune responses.
Copyright © 2019 Cecilia Gomes Barbosa et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Memórias do Instituto Oswaldo Cruz. 1909;1(2):159–218. doi: 10.1590/S0074-02761909000200008. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

