Functional Outcomes of Cerebellar Malformations
- PMID: 31636540
- PMCID: PMC6787289
- DOI: 10.3389/fncel.2019.00441
Functional Outcomes of Cerebellar Malformations
Abstract
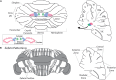
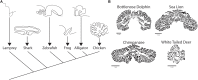
The cerebellum is well-established as a primary center for controlling sensorimotor functions. However, recent experiments have demonstrated additional roles for the cerebellum in higher-order cognitive functions such as language, emotion, reward, social behavior, and working memory. Based on the diversity of behaviors that it can influence, it is therefore not surprising that cerebellar dysfunction is linked to motor diseases such as ataxia, dystonia, tremor, and Parkinson's disease as well to non-motor disorders including autism spectrum disorders (ASD), schizophrenia, depression, and anxiety. Regardless of the condition, there is a growing consensus that developmental disturbances of the cerebellum may be a central culprit in triggering a number of distinct pathophysiological processes. Here, we consider how cerebellar malformations and neuronal circuit wiring impact brain function and behavior during development. We use the cerebellum as a model to discuss the expanding view that local integrated brain circuits function within the context of distributed global networks to communicate the computations that drive complex behavior. We highlight growing concerns that neurological and neuropsychiatric diseases with severe behavioral outcomes originate from developmental insults to the cerebellum.
Keywords: Purkinje cell; cerebellar nuclei; cerebellum; circuitry; cognitive; development; motor.
Copyright © 2019 Gill and Sillitoe.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

