Naturally Acquired Antibody Response to Malaria Transmission Blocking Vaccine Candidate Pvs230 Domain 1
- PMID: 31636633
- PMCID: PMC6788386
- DOI: 10.3389/fimmu.2019.02295
Naturally Acquired Antibody Response to Malaria Transmission Blocking Vaccine Candidate Pvs230 Domain 1
Abstract
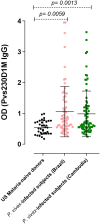
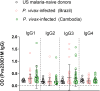
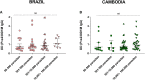
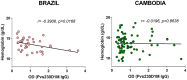
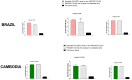
Plasmodium vivax malaria incidence has increased in Latin America and Asia and is responsible for nearly 74.1% of malaria cases in Latin America. Immune responses to P. vivax are less well characterized than those to P. falciparum, partly because P. vivax is more difficult to cultivate in the laboratory. While antibodies are known to play an important role in P. vivax disease control, few studies have evaluated responses to P. vivax sexual stage antigens. We collected sera or plasma samples from P. vivax-infected subjects from Brazil (n = 70) and Cambodia (n = 79) to assess antibody responses to domain 1 of the gametocyte/gamete stage protein Pvs230 (Pvs230D1M). We found that 27.1% (19/70) and 26.6% (21/79) of subjects from Brazil and Cambodia, respectively, presented with detectable antibody responses to Pvs230D1M antigen. The most frequent subclasses elicited in response to Pvs230D1M were IgG1 and IgG3. Although age did not correlate significantly with Pvs230D1M antibody levels overall, we observed significant differences between age strata. Hemoglobin concentration inversely correlated with Pvs230D1M antibody levels in Brazil, but not in Cambodia. Additionally, we analyzed the antibody response against Pfs230D1M, the P. falciparum ortholog of Pvs230D1M. We detected antibodies to Pfs230D1M in 7.2 and 16.5% of Brazilian and Cambodian P. vivax-infected subjects. Depletion of Pvs230D1M IgG did not impair the response to Pfs230D1M, suggesting pre-exposure to P. falciparum, or co-infection. We also analyzed IgG responses to sporozoite protein PvCSP (11.4 and 41.8% in Brazil and Cambodia, respectively) and to merozoite protein PvDBP-RII (67.1 and 48.1% in Brazil and Cambodia, respectively), whose titers also inversely correlated with hemoglobin concentration only in Brazil. These data establish patterns of seroreactivity to sexual stage Pvs230D1M and show similar antibody responses among P. vivax-infected subjects from regions of differing transmission intensity in Brazil and Cambodia.
Keywords: Plasmodium vivax; Pvs230; malaria; seroreactivity; transmission-blocking vaccine.
Copyright © 2019 Tentokam, Amaratunga, Alani, MacDonald, Narum, Salinas, Kwan, Suon, Sreng, Pereira, Tolia, Fujiwara, Bueno, Duffy and Coelho.
Figures



Similar articles
-
Distinct immunogenicity outcomes of DNA vaccines encoding malaria transmission-blocking vaccine target antigens Pfs230D1M and Pvs230D1.Vaccine. 2025 Feb 15;47:126696. doi: 10.1016/j.vaccine.2024.126696. Epub 2025 Jan 8. Vaccine. 2025. PMID: 39787798
-
Plasmodium vivax gametocyte protein Pvs230 is a transmission-blocking vaccine candidate.Vaccine. 2012 Feb 27;30(10):1807-12. doi: 10.1016/j.vaccine.2012.01.003. Epub 2012 Jan 11. Vaccine. 2012. PMID: 22245309
-
Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population.Am J Trop Med Hyg. 2005 Aug;73(2):244-55. Am J Trop Med Hyg. 2005. PMID: 16103583
-
The Duffy binding protein as a key target for a Plasmodium vivax vaccine: lessons from the Brazilian Amazon.Mem Inst Oswaldo Cruz. 2014 Aug;109(5):608-17. doi: 10.1590/0074-0276130592. Mem Inst Oswaldo Cruz. 2014. PMID: 25185002 Free PMC article. Review.
-
Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites.Immunol Rev. 2020 Jan;293(1):190-215. doi: 10.1111/imr.12828. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31840844 Free PMC article. Review.
Cited by
-
Evaluation of the transmission-blocking potential of Plasmodium vivax antigen Pvg37 using transgenic rodent parasites and clinical isolates.Front Cell Infect Microbiol. 2025 Jan 24;15:1529770. doi: 10.3389/fcimb.2025.1529770. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 39925376 Free PMC article.
-
Distinct immunogenicity outcomes of DNA vaccines encoding malaria transmission-blocking vaccine target antigens Pfs230D1M and Pvs230D1.Vaccine. 2025 Feb 15;47:126696. doi: 10.1016/j.vaccine.2024.126696. Epub 2025 Jan 8. Vaccine. 2025. PMID: 39787798
-
Exploring the naturally acquired response to Pvs47 gametocyte antigen.Front Immunol. 2024 Oct 10;15:1455454. doi: 10.3389/fimmu.2024.1455454. eCollection 2024. Front Immunol. 2024. PMID: 39450180 Free PMC article.
-
Longitudinal analysis of antibody responses to Plasmodium vivax sporozoite antigens following natural infection.PLoS Negl Trop Dis. 2024 Jan 26;18(1):e0011907. doi: 10.1371/journal.pntd.0011907. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38277340 Free PMC article.
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
References
-
- WHO World Malaria Report. Geneva: (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

