A MEMS ultrasound stimulation system for modulation of neural circuits with high spatial resolution in vitro
- PMID: 31636922
- PMCID: PMC6799809
- DOI: 10.1038/s41378-019-0070-5
A MEMS ultrasound stimulation system for modulation of neural circuits with high spatial resolution in vitro
Abstract
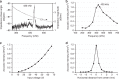
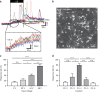
Neuromodulation by ultrasound has recently received attention due to its noninvasive stimulation capability for treating brain diseases. Although there have been several studies related to ultrasonic neuromodulation, these studies have suffered from poor spatial resolution of the ultrasound and low repeatability with a fixed condition caused by conventional and commercialized ultrasound transducers. In addition, the underlying physics and mechanisms of ultrasonic neuromodulation are still unknown. To determine these mechanisms and accurately modulate neural circuits, researchers must have a precisely controllable ultrasound transducer to conduct experiments at the cellular level. Herein, we introduce a new MEMS ultrasound stimulation system for modulating neurons or brain slices with high spatial resolution. The piezoelectric micromachined ultrasonic transducers (pMUTs) with small membranes (sub-mm membranes) generate enough power to stimulate neurons and enable precise modulation of neural circuits. We designed the ultrasound transducer as an array structure to enable localized modulation in the target region. In addition, we integrated a cell culture chamber with the system to make it compatible with conventional cell-based experiments, such as in vitro cell cultures and brain slices. In this work, we successfully demonstrated the functionality of the system by showing that the number of responding cells is proportional to the acoustic intensity of the applied ultrasound. We also demonstrated localized stimulation capability with high spatial resolution by conducting experiments in which cocultured cells responded only around a working transducer.
Keywords: Electrical and electronic engineering; Microfluidics.
© The Author(s) 2019.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
