Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome
- PMID: 31637019
- PMCID: PMC6799818
- DOI: 10.1038/s41392-019-0074-5
Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome
Abstract
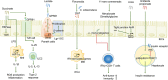
The trillions of microorganisms in the gut microbiome have attracted much attention recently owing to their sophisticated and widespread impacts on numerous aspects of host pathophysiology. Remarkable progress in large-scale sequencing and mass spectrometry has increased our understanding of the influence of the microbiome and/or its metabolites on the onset and progression of extraintestinal cancers and the efficacy of cancer immunotherapy. Given the plasticity in microbial composition and function, microbial-based therapeutic interventions, including dietary modulation, prebiotics, and probiotics, as well as fecal microbial transplantation, potentially permit the development of novel strategies for cancer therapy to improve clinical outcomes. Herein, we summarize the latest evidence on the involvement of the gut microbiome in host immunity and metabolism, the effects of the microbiome on extraintestinal cancers and the immune response, and strategies to modulate the gut microbiome, and we discuss ongoing studies and future areas of research that deserve focused research efforts.
Keywords: Cancer; Cancer metabolism; Cancer therapy.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources

