Wound Closure Outcomes Suggest Clinical Equivalency Between Lyopreserved and Cryopreserved Placental Membranes Containing Viable Cells
- PMID: 31637101
- PMCID: PMC6798798
- DOI: 10.1089/wound.2019.1028
Wound Closure Outcomes Suggest Clinical Equivalency Between Lyopreserved and Cryopreserved Placental Membranes Containing Viable Cells
Abstract
Objective: To evaluate the clinical outcomes of lyopreserved placental membrane containing viable cells (vLPM) in the treatment of nonhealing wounds of various etiologies, and to compare them to those previously reported for cryopreserved placental membrane containing viable cells (vCPM). Approach: Patients with nonhealing wounds who qualified to receive advanced wound therapies were consecutively enrolled and treated weekly with vLPM plus standard of care (SOC) at five centers. Data were de-identified and retrospectively analyzed. Outcomes included closure, time to closure, number of vLPM applications, and adverse events (AEs). Results: Seventy-eight patients with 98 wounds (41 diabetic foot ulcers [DFUs], 19 venous leg ulcers [VLUs], 10 surgical, and 28 others) with an average size of 13.3 cm2 and 8.7 months duration were treated. Fifty-eight of the 98 wounds (59.2%) achieved complete closure with median time to closure of 63 days and 6 vLPM applications. The closure by wound etiology was 63% for DFUs, 47% for VLUs, 70% for surgical wounds, and 57% for other types of wounds. Similar closure rates have been previously demonstrated for vCPM. Wound duration was the main predictor of closure: 65.8% versus 30.0% (p = 0.004) closure was achieved for wounds of ≤12 and >12 months duration, respectively. There were no AEs related to vLPM application. Innovation: This is the first multicenter case series evaluating the clinical outcomes of vLPM in a real-world setting. Conclusion: These results support clinical equivalency between the two placental membrane formulations with the added convenience of room-temperature storage for vLPM, allowing it to be used in any wound-care setting.
Keywords: lyopreserved; nonhealing; placental membrane; viable; wound.
© Charles E. Ananian, et al. 2019; Published by Mary Ann Liebert, Inc.
Figures

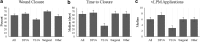
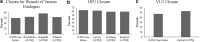

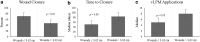
References
-
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research; Center for Devices and Radiological Health. Guidance for industry: chronic cutaneous ulcer and burn wounds—developing products for treatment. 2006. June https://www.fda.gov/downloads/drugs/guidances/ucm071324.pdf (last accessed March27, 2019)
-
- Climov M, Bayer LR, Moscoso AV, et al. The role of dermal matrices in treating inflammatory and diabetic wounds. Plast Reconstr Surg 2016;138(3Suppl):148S–157S - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
