Interventions for preventing bone disease in kidney transplant recipients
- PMID: 31637698
- PMCID: PMC6803293
- DOI: 10.1002/14651858.CD005015.pub4
Interventions for preventing bone disease in kidney transplant recipients
Abstract
Background: People who have chronic kidney disease (CKD) have important changes to bone structure, strength, and metabolism. Children experience bone deformity, pain, and delayed or impaired growth. Adults experience limb and vertebral fractures, avascular necrosis, and pain. The fracture risk after kidney transplantation is four times that of the general population and is related to Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) occurring with end-stage kidney failure, steroid-induced bone loss, and persistent hyperparathyroidism after transplantation. Fractures may reduce quality of life and lead to being unable to work or contribute to community roles and responsibilities. Earlier versions of this review have found low certainty evidence for effects of treatment. This is an update of a review first published in 2005 and updated in 2007.
Objectives: This review update evaluates the benefits and harms of interventions for preventing bone disease following kidney transplantation.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 16 May 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: RCTs and quasi-RCTs evaluating treatments for bone disease among kidney transplant recipients of any age were eligible.
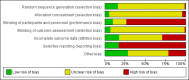
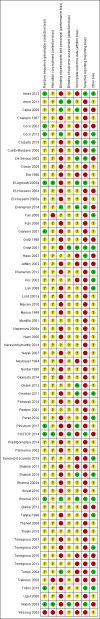
Data collection and analysis: Two authors independently assessed trial risks of bias and extracted data. Statistical analyses were performed using random effects meta-analysis. The risk estimates were expressed as a risk ratio (RR) for dichotomous variables and mean difference (MD) for continuous outcomes together with the corresponding 95% confidence interval (CI). The primary efficacy outcome was bone fracture. The primary safety outcome was acute graft rejection. Secondary outcomes included death (all cause and cardiovascular), myocardial infarction, stroke, musculoskeletal disorders (e.g. skeletal deformity, bone pain), graft loss, nausea, hyper- or hypocalcaemia, kidney function, serum parathyroid hormone (PTH), and bone mineral density (BMD).
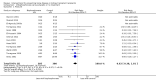
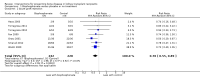
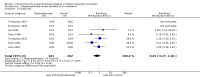
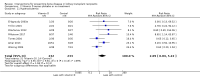
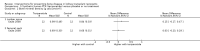
Main results: In this 2019 update, 65 studies (involving 3598 participants) were eligible; 45 studies contributed data to our meta-analyses (2698 participants). Treatments included bisphosphonates, vitamin D compounds, teriparatide, denosumab, cinacalcet, parathyroidectomy, and calcitonin. Median duration of follow-up was 12 months. Forty-three studies evaluated bone density or bone-related biomarkers, with more recent studies evaluating proteinuria and hyperparathyroidism. Bisphosphonate therapy was usually commenced in the perioperative transplantation period (within 3 weeks) and regardless of BMD. Risks of bias were generally high or unclear leading to lower certainty in the results. A single study reported outcomes among 60 children and adolescents. Studies were not designed to measure treatment effects on fracture, death or cardiovascular outcomes, or graft loss.Compared to placebo, bisphosphonate therapy administered over 12 months in transplant recipients may prevent fracture (RR 0.62, 95% CI 0.38 to 1.01; low certainty evidence) although the 95% CI included the possibility that bisphosphonate therapy might make little or no difference. Fracture events were principally vertebral fractures identified during routine radiographic surveillance. It was uncertain whether any other drug class decreased fracture (low or very low certainty evidence). It was uncertain whether interventions for bone disease in kidney transplantation reduce all-cause or cardiovascular death, myocardial infarction or stroke, or graft loss in very low certainty evidence. Bisphosphonate therapy may decrease acute graft rejection (RR 0.70, 95% CI 0.55 to 0.89; low certainty evidence), while it is uncertain whether any other treatment impacts graft rejection (very low certainty evidence). Bisphosphonate therapy may reduce bone pain (RR 0.20, 95% CI 0.04 to 0.93; very low certainty evidence), while it was very uncertain whether bisphosphonates prevent spinal deformity or avascular bone necrosis (very low certainty evidence). Bisphosphonates may increase to risk of hypocalcaemia (RR 5.59, 95% CI 1.00 to 31.06; low certainty evidence). It was uncertain whether vitamin D compounds had any effect on skeletal, cardiovascular, death, or transplant function outcomes (very low certainty or absence of evidence). Evidence for the benefits and harms of all other treatments was of very low certainty. Evidence for children and young adolescents was sparse.
Authors' conclusions: Bisphosphonate therapy may reduce fracture and bone pain after kidney transplantation, however low certainty in the evidence indicates it is possible that treatment may make little or no difference. It is uncertain whether bisphosphonate therapy or other bone treatments prevent other skeletal complications after kidney transplantation, including spinal deformity or avascular bone necrosis. The effects of bone treatment for children and adolescents after kidney transplantation are very uncertain.
Conflict of interest statement
Suetonia C Palmer: none known
Edmund YM Chung: none known
David O McGregor: none known
Friederike Bachmann: none known
Giovanni FM Strippoli: none known
Figures









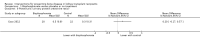










































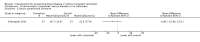





















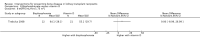











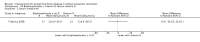
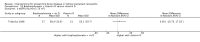

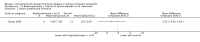













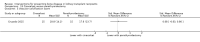















Update of
References
References to studies included in this review
Amer 2013 {published data only}
-
- Amer H, Griffin M, Cosio F, Park W, Kremers W, Mazur M, et al. Oral paricalcitol in kidney transplant recipients receiving a corticosteroid‐free immunosuppressive regimen: an open label randomized trial [abstract no: LB33]. American Journal of Transplantation 2012;12(Suppl S3):452. [EMBASE: 70747416]
-
- Amer H, Griffin M, Park W, Kremers W, Stegall M, Cosio F, et al. Effects of paricalcitol on bone turnover in kidney transplant recipients [abstract no: A39]. Transplantation 2014;98(Suppl 1):882. [EMBASE: 71546547]
-
- Amer H, Griffin MD, Stegall MD, Cosio FG, Park WD, Kremers WK, et al. Oral paricalcitol reduces the prevalence of posttransplant hyperparathyroidism: results of an open label randomized trial. American Journal of Transplantation 2013;13(6):1576‐85. [MEDLINE: ] - PubMed
Arnol 2011 {published data only}
-
- Arnol M, Oblak M, Mlinsek G, Bren AF, Buturovic‐Ponikvar J, Kandus A. Proteinuria in kidney transplant recipients: prevalence in a national cohort and study design of the effect of paricalcitol for reduction of proteinuria [abstract no: RO‐204]. Transplant International 2011;24(Suppl 2):185. [EMBASE: 70527733]
-
- Arnol M, Oblak M, Mlinsek G, Buturovic‐Ponikvar J, Kandus A. Paricalcitol versus placebo for reduction of proteinuria in kidney transplant recipients: a double‐blind, randomized controlled trial [abstract no: 426.4]. Transplantation 2016;100(7 Suppl 1):S214‐5. [EMBASE: 613005120] - PubMed
Cejka 2008 {published data only}
-
- Cejka D, Benesch T, Krestan C, Roschger P, Klaushofer K, Pietschmann P, et al. Effect of teriparatide on early bone loss after kidney transplantation. American Journal of Transplantation 2008;8(9):1864‐70. [MEDLINE: ] - PubMed
Chalopin 1987 {published data only}
-
- Chalopin JM, Garnier PC, D'Athis P, Cabanne JF, Perceau C, Tanter Y, et al. Vitamin D3 metabolites and mineral metabolism parameters after renal transplantation: prospective randomized study with 250H vitamin D3 therapy [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:418. [CENTRAL: CN‐00784682]
Coco 2003 {published data only}
-
- Coco M, Glicklich D, Bognar I, Burris L, Durkin P, Tellis V, et al. Randomized trial of IV pamidronate to ameliorate bone loss in renal transplant (RT) recipients [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):927A. [CENTRAL: CN‐00550697] - PubMed
-
- Coco M, Glicklich D, Burris L, Bognar I, Tellis V, Greenstein S, et al. Prospective randomised trial of IV pamidronate in renal transplant recipients: 6 and 12 month follow‐up [abstract no: F‐FC044]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):10A. [CENTRAL: CN‐00444866] - PubMed
-
- Coco M, Glicklich D, Burris L, Durkin P, Tellis V, Greenstein S, et al. Effect of IV pamidronate on bone biochemical parameters in renal transplant recipients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):561A. [CENTRAL: CN‐00550699]
-
- Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. Journal of the American Society of Nephrology 2003;14(10):2669‐76. [MEDLINE: ] - PubMed
-
- Lee S, Glicklich D, Coco M. Pamidronate used to ameliorate post renal transplant bone loss is not associated with increased renal dysfunction [abstract no: SU‐PO729]. Journal of the American Society of Nephrology 2003;14(Nov):695A. [CENTRAL: CN‐00615885]
Coco 2012 {published data only}
-
- Coco M, Glicklich D, Pullman J. Prophylactic risedronate in new renal transplant recipients: effects on serial bone mineral density and bone histology [abstract no: F‐PO909]. Journal of the American Society of Nephrology 2007;18(Abstracts):302A. [CENTRAL: CN‐00783952]
-
- Coco M, Glicklich D, Pullman J. Randomized controlled trial of risedronate in renal transplant recipients: histomorphometry shows lowered bone turnover but not adynamic bone [Abstract no: SA‐PO2830]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):759A.
Cruzado 2015 {published data only}
-
- Cruzado J, Torregrosa V, Mast R, Garcia‐Barrasa A, Bestard O, Melilli E, et al. A randomized study comparing parathyroidectomy versus cinacalcet to treat hypercalcemia in kidney allograft recipients with persistent hyperparathyroidism [abstract no: 756]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71952932]
-
- Cruzado JM, Moreno P, Torregrosa JV, Taco O, Mast R, Gomez‐Vaquero C, et al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. Journal of the American Society of Nephrology 2015;27(8):2487‐94. [MEDLINE: ] - PMC - PubMed
-
- Cruzado JM, Taco O, Vicens T, Mast R, Polo C, Bestard O, et al. A randomized study comparing parathyroidectomy versus cinacalcet to treat hypercalcemia in kidney allograft recipients with persistent hyperparathyroidism [abstract]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii36. [EMBASE: 72206357]
Cueto‐Manzano 2000 {published data only}
-
- Cueto‐Manzano AM, Konel S, Freemont AJ, Adams JE, Mawer B, Gokal R, et al. Effect of 1,25‐dihydroxyvitamin D3 and calcium carbonate on bone loss associated with long‐term renal transplantation. American Journal of Kidney Diseases 2000;35(2):227‐36. [MEDLINE: ] - PubMed
De Sevaux 2002 {published data only}
-
- Sevaux R, Hoitsma A, Corstens F, Wetzels J. Prophylaxis of bone loss after renal transplantation with calcium and vitamin D [abstract no: A4855]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):929A. [CENTRAL: CN‐00550384]
-
- Sevaux RG, Hoitsma AJ, Corstens FHM, Wetzels JF. Treatment with vitamin D and calcium reduces bone loss after renal transplantation: a randomized study. Journal of the American Society of Nephrology 2002;13(6):1608‐14. [MEDLINE: ] - PubMed
Dovas 2009 {published data only}
-
- Dovas S, Economidou D, Papagianni A, Kazantzidou E, Pateinakis P, Efstratiadis G, et al. A prospective study for prevention of post transplantation bone loss in renal transplant recipients [abstract no: P‐662]. Transplant International 2009;22(Suppl 2):259.
Eid 1996 {published data only}
-
- Eid P, Scuderi G, Aroldi A, Ciammella M, Nencioni T. Bone loss after renal transplantation: HRT versus calcitriol in postmenopausal recipients [abstract no: PTu844]. Osteoporosis International 1996;6(Suppl 1):294. [CENTRAL: CN‐00260191]
El‐Agroudy 2003a {published data only}
-
- El‐Agroudy A, El‐Husseini A, El‐Sayed M, Ghoneim MA. Prevention of osteoporosis after kidney transplantation: a prospective randomized study [abstract no: M777]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):249.
-
- El‐Agroudy AE, El‐Husseini AA, El‐Sayed M, Ghoneim MA. Preventing bone loss in renal transplant recipients with vitamin D. Journal of the American Society of Nephrology 2003;14(11):2975‐9. [MEDLINE: ] - PubMed
-
- El‐Agroudy AE, El‐Husseini AA, El‐Sayed M, Mohsen T, Ghoneim MA. A prospective randomized study for prevention of postrenal transplantation bone loss. Kidney International 2005;67(5):2039‐45. [MEDLINE: ] - PubMed
El‐Husseini 2004 {published data only}
-
- Husseini A, Elagroudy A, Elsayed M, Sobh M, Ghoneim M. Treatment of bone loss with vitamin D in live related kidney transplant children and adolescents: a prospective randomized study [abstract no: SP231]. 41st Congress European Renal Association European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:93. [CENTRAL: CN‐00509176]
-
- El‐Husseini A, El‐Agroudy A, El‐Sayed M, Sobh M, Ghoneim M. Treatment of bone loss in renal transplant children and adolescents [abstract no: T818]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):544. [CENTRAL: CN‐00445216]
-
- El‐Husseini AA, El‐Agroudy AE, El‐Sayed M, Sobh MA, Ghoneim MA. A prospective randomized study for the treatment of bone loss with vitamin D during kidney transplantation in children and adolescents. American Journal of Transplantation 2004;4(12):2052‐7. [MEDLINE: ] - PubMed
-
- El‐Husseini AA, El‐Agroudy AE, El‐Sayed MF, Sobh MA, Ghoneim MA. A prospective randomized study for the treatment of bone loss with alendronate in kidney transplant children and adolescents [abstract no: SP181]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v80. [CENTRAL: CN‐00782294]
-
- El‐Husseini AA, El‐Agroudy AE, El‐Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatric Transplantation 2004;8(4):357‐61. [MEDLINE: ] - PubMed
El‐Husseini 2005a {published data only}
-
- El‐Husseini AA, El‐Agroudy A, El‐Sayed MF, Sobh MA, Ghoneim MA. Alfacalcidol versus calcitonin for the treatment of bone loss after renal transplantation in children and adolescents [abstract no: MP482]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v361. [CENTRAL: CN‐00782136]
Evenepoel 2014 {published data only}
-
- Evenepoel P, Cooper K, Holdaas H, Messa P, Mourad G, Olgaard K, et al. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. American Journal of Transplantation 2014;14(11):2545‐55. [MEDLINE: ] - PubMed
Fan 2000 {published data only}
-
- Fan S, Almond MK, Ball E, Evans K, Cunningham J. Randomized prospective study demonstrating prevention of bone loss by pamidronate during the first year after renal transplantation [abstract no: A2714]. Journal of the American Society of Nephrology 1996;7(Program & Abstracts):1789. [CENTRAL: CN‐00445275]
-
- Fan SL, Almond MK, Ball E, Evans K, Cunningham J. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney International 2000;57(2):684‐90. [MEDLINE: ] - PubMed
-
- Fan SL, Kumar S, Cunningham J. Long‐term effects on bone mineral density of pamidronate given at the time of renal transplantation. Kidney International 2003;63(6):2275‐9. [MEDLINE: ] - PubMed
Fujii 2006 {published data only}
-
- Fujii N, Hamano T, Mikami S, Ito T, Kokado Y, Kojima Y, et al. Oral risedronate is a successful treatment for bone loss in the long‐term kidney transplant patients [abstract no: TH‐PO123]. Journal of the American Society of Nephrology 2006;17(Abstracts):132A. [CENTRAL: CN‐00783786]
Giannini 2001 {published data only}
-
- Carraro G, Perin N, Giannini S, Naso A, Legnaro A, D'Angelo A, et al. Alendronate prevents further bone loss in renal transplant recipients [abstract no: 448]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisbon, Portugal. 2001.
-
- Giannini S, D'Angelo A, Carraro G, Nobile M, Rigotti P, Bonfante L, et al. Alendronate prevents further bone loss in renal transplant recipients. Journal of Bone & Mineral Research 2001;16(11):2111‐7. [MEDLINE: ] - PubMed
Grotz 1998 {published data only}
-
- Grotz WH, Rump LC, Niessen A, Schmidt‐Gayk H, Reichelt A, Gunter K, et al. Treatment of osteopenia and osteoporosis after kidney transplantation. Transplantation 1998;66(8):1004‐8. [MEDLINE: ] - PubMed
Grotz 2001 {published data only}
-
- Gerke P, Olschewski M, Grotz W. Early ibandronate administration improves long‐term renal allograft function [abstract no: SA‐PO2533]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):679A. [CENTRAL: CN‐00782904]
-
- Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, et al. Effect of ibandronate on bone loss and renal function after kidney transplantation. Journal of the American Society of Nephrology 2001;12(7):1530‐7. [MEDLINE: ] - PubMed
Haas 2003 {published data only}
-
- Haas M, Leko‐Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Mitterbauer C, et al. Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney International 2003;63(3):1130‐6. [MEDLINE: ] - PubMed
-
- Schwarz C, Mitterbauer C, Heinze G, Woloszczuk W, Haas M, Oberbauer R. Nonsustained effect of short‐term bisphosphonate therapy on bone turnover three years after renal transplantation. Kidney International 2004;65(1):304‐9. [MEDLINE: ] - PubMed
-
- Schwarz C, Mitterbauer C, Heinze G, Wolusczcuk W, Haas M, Oberbauer R. Non‐sustained effect of short term bisphsophonate therapy on bone turnover three years after renal transplantation [abstract no: SU‐PO736]. Journal of the American Society of Nephrology 2003;14(Nov):697A. [CENTRAL: CN‐00783721]
Jeffery 2003 {published data only}
-
- Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. A randomized prospective trial of calcitriol versus alendronate for the treatment of osteopenia in renal transplant recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):353. [CENTRAL: CN‐00445907] - PubMed
-
- Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation 2003;76(10):1498‐502. [MEDLINE: ] - PubMed
Kharlamov 2012 {published data only}
-
- Kharlamov AN, Parrish AN, Ivanova EJ, Gabinsky JL, Veselova VS, Novoselova OS. Cholecalciferol for prevention of chronic allograft nephropathy, kidney repair, and management of cardiorenal syndrome in vitamin D insufficient kidney transplant recipients [abstract]. European Journal of Medical Research 2010;15(Suppl 1):124. [EMBASE: 71295070]
-
- Kharlamov AN, Perrish AN, Gabiskii I, Ronne K, Ivanova EI. Vitamin D in the treatment of cardiorenal syndrome in patients with chronic nephropathy. Kardiologiia 2012;52(3):33‐44. [MEDLINE: ] - PubMed
Koc 2002 {published data only}
-
- Koc M, Tuglular H, Arikan H, Ozener C, Akoglu E. Alendronate increases bone mineral density in long‐term renal transplant recipients. Transplantation Proceedings 2002;34(6):2111‐3. [MEDLINE: ] - PubMed
-
- Koc M, Tuglular S, Arikan H, Ozener C, Akoglu E. Alendronate increases bone mineral density in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A227. [CENTRAL: CN‐00446130] - PubMed
Lan 2008 {published data only}
-
- Lan G, Peng L, Xie X, Peng F, Wang Y, Yu S. Alendronate is effective to treat bone loss in renal transplantation recipients. Transplantation Proceedings 2008;40(10):3496‐8. [MEDLINE: ] - PubMed
Lord 2001a {published data only}
-
- Lord H, Cailhier J, Leblanc M, Boucher A, Dandavino R, Ouimet D, et al. Prevention of bone loss following kidney transplantation with alendronate: a randomized controlled study [abstract no: A4897]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):937‐8A. [CENTRAL: CN‐00783925]
Marcen 2010 {published data only}
-
- Marcén R, Fernández‐Rodriguez A, Galeano C, Villacorta FJ, Villafruela JJ, Teruel JL, et al. Cholecalciferol supplements in renal transplant recipients with vitamin D deficiency [abstract no: 1688]. American Journal of Transplantation 2010;10(Suppl 4):518. [EMBASE: 70465063] - PubMed
Messa 1999 {published data only}
-
- Messa P, Sindici C, Miotti V, Gropuzzo M, Cattaruzzi E, Englaro E, et al. Calcitriol and VDR genotype influences on bone mineral density after renal transplantation [abstract no: A3149]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):622A. [CENTRAL: CN‐00782465]
Montilla 2001 {published data only}
-
- Montilla C, Carlini RG, Martinis R, Hernandez E, Arminio A, Clesca P, et al. One year controlled trial of pamidronate in the treatment of severe osteopenia after long term renal transplantation (TX) [abstract no: A4914]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):941A. [CENTRAL: CN‐00783760]
Nakamura 2009a {published data only}
-
- Nakamura N, Ogahara S, Mikami H, Matsubara T, Higuchi K, Furuya R, et al. The effects of bisphosphonates to prevent GIOP in kidney transplantation recipients. The tentative report of single‐blind randomized prospective clinical study [abstract no: P‐568]. Transplant International 2009;22(Suppl 2):235‐6.
Nam 2000 {published data only}
-
- Nam JH, Moon JI, Chung SS, Kim SI, Park KI, Song YD, et al. Pamidronate and calcitriol trial for the prevention of early bone loss after renal transplantation. Transplantation Proceedings 2000;32(7):1876. [MEDLINE: ] - PubMed
Narasimhamurthy 2014 {published data only}
-
- Narasimhamurthy M, Kamal F, Igari M, Natraj S, Gohh R. Reduction of proteinuria with selective vitamin D receptor activation using paricalcitol in renal transplant patients [abstract no: 2244]. Transplantation 2014;98(Suppl 1):127. [EMBASE: 71543930]
Nayak 2007 {published data only}
-
- Nayak B, Guleria S, Varma M, Tandon N, Aggarwal S, Bhowmick D, et al. Effect of bisphosphonates on bone mineral density after renal transplantation as assessed by bone mineral densitometry. Transplantation Proceedings 2007;39(3):750‐2. [MEDLINE: ] - PubMed
Neubauer 1984 {published data only}
-
- Neubauer E, Neubauer N, Ritz E, Dreikorn K, Krause KH. Bone mineral content after renal transplantation. Placebo‐controlled prospective study with 1,25‐dihydroxy vitamin D3. Klinische Wochenschrift 1984;62(2):93‐6. [MEDLINE: ] - PubMed
Nordal 1995 {published data only}
-
- Nordal KP, Halse J, Dahl E. The effect of nasal calcitonin on bone mineral density in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 1996;11(6):1212. [CENTRAL: CN‐00261230]
-
- Nordal KP, Halse J, Kronborg J, Dahl E, Hartmann A. Nasal calcitonin reduces bone loss in renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1995;6(3):1109. [CENTRAL: CN‐00485256]
Okamoto 2014 {published data only}
Oliden 2012 {published data only}
-
- Oliden C, Leon L, Tavera M, Minue E, Anzieta N, Colom P, et al. Oral paricalcitol versus oral calcitriol for the treatment of secondary hyperparathyroidism in renal transplantation [abstract no: 1707]. Transplantation 2012;94(10 Suppl):898. [EMBASE: 71251572]
Omidvar 2011 {published data only}
-
- Omidvar B, Ghorbani A, Shahbazian H, Beladi Mousavi SS, Shariat Nabavi SJ, Alasti M. Comparison of alendronate and pamidronate on bone loss in kidney transplant patients for the first 6 months of transplantation. [Erratum appears in Iran J Kidney Dis. 2012 Jul;6(4):321]. Iranian Journal of Kidney Diseases 2011;5(6):420‐4. [MEDLINE: ] - PubMed
-
- Omidvar B, Ghorbani A, Shahbazian H, Mousavi SS, Hayati F, Nabavi SJ. Comparison the effect of alendronate and pamidronate on early BMD changes in kidney transplant patients in the first 6 months of transplantation [abstract no: 0740]. International Journal of Rheumatic Diseases 2010;13(Suppl 1):170. [EMBASE: 70198173]
Pasquali 2014 {published data only}
-
- Pasquali M, Tartaglione L, Rotondi S, Luisa MM, Mandanici G, Leonangeli C, et al. Cinacalcet (CM) vs paricalcitol (PC) in renal transplant (TX) patients with hypercalcemic persisting secondary hyperparathyroidism (HPSH) a pilot study (EudraCT 2010‐021041‐42) [abstract no: MP219]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii400. [EMBASE: 71492622]
Peeters 2001 {published data only}
-
- Peeters J, Maes B, Vanwalleghem J, Boonen S, Vanrenterghem Y. Alfa‐calcidol protects against spinal bone loss after cadaveric renal transplantation: a randomised placebo‐controlled trial [abstract no: O14]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):6‐7. [CENTRAL: CN‐00782134]
-
- Peeters JC, Maes BD, Herelixka AS, Vanwalleghem JF, Boonen S, Vanrenterghem YF. Alfa‐calcidol protects against spinal bone loss after renal transplantation: a randomised placebo controlled trial [abstract no: A4925]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):943A. [CENTRAL: CN‐00782135]
Perez 2010 {published data only}
-
- Perez V, Sanchez A, Bayes B, Navarro‐Munoz M, Lauzurica R, Pastor MC, et al. Effect of paricalcitol on the urinary peptidome of kidney transplant patients. Transplantation Proceedings 2010;42(8):2924‐7. [MEDLINE: ] - PubMed
-
- Perez V, Sanchez‐Escuredo A, Lauzurica R, Bayes B, Navarro‐Munoz M, Pastor MC, et al. Magnetic bead‐based proteomic technology to study paricalcitol effect in kidney transplant recipients. European Journal of Pharmacology 2013;709(1‐3):72‐9. [MEDLINE: ] - PubMed
Pihlstrom 2017 {published data only}
-
- Pihlstrom HK, Gatti F, Hammarstrom C, Eide IA, Kasprzycka M, Wang J, et al. Early introduction of oral paricalcitol in renal transplant recipients. An open‐label randomized study. Transplant International 2017;30(8):827‐40. [MEDLINE: ] - PubMed
-
- Pihlstrøm H, Eide IA, Midtvedt K, Hartmann A, Bollerslev J, Svensson MHS. Paricalcitol for prevention and reduction of albuminuria in the general renal transplant recipient [abstract no: P767]. Transplant International 2015;28(Suppl 4):815. [EMBASE: 72112694]
-
- Pihlstrøm H, Gatti F, Hammarstrom C, Eide I, Kasprzycka M, Wang J, et al. Early introduction of oral paricalcitol in renal transplant recipients. an open‐label randomized study [abstract no: D149]. American Journal of Transplantation 2017;17(Suppl 3):760. [EMBASE: 615705633] - PubMed
POSTOP 2014 {published data only}
-
- Bonani M, Brockmann J, Cohen CD, Fehr T, Nocito A, Schiesser M, et al. A randomized open‐label clinical trial examining the effect of denosumab on the prevention of 1st‐year bone mineral density loss after renal transplantation (POSTOP study; NCT01377467) [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii304. [EMBASE: 70766189]
-
- Bonani M, Fehr T, Mueller T, Blum M, Brockmann J, Frey D, et al. Prevention of bone mineral density (BMD) loss after kidney transplantation with the rank ligand inhibitor denosumab (POSTOP study): Baseline data, biomarker response and initial safety [abstract no: P47]. Swiss Medical Weekly 2014;144(Suppl 208):24S. [EMBASE: 71713909]
-
- Bonani M, Frey D, Brockmann J, Fehr T, Mueller TF, Saleh L, et al. Effect of twice‐yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: a randomized controlled trial. American Journal of Transplantation 2016;16(6):1882‐91. [MEDLINE: ] - PubMed
-
- Bonani M, Frey D, Brockmann J, Fehr T, Muller T, Graf N, et al. Denosumab prevents bone mineral density loss in de novo kidney transplant recipients: results from a randomized controlled trial [abstract]. American Journal of Transplantation 2016;16(Suppl 3):323. [EMBASE: 611700418] - PubMed
-
- Bonani M, Frey D, Brockmann J, Fehr T, Muller T, Graf N, et al. Infections in de novo kidney transplant recipients treated with the RANKL inhibitor denosumab: a post‐hoc analysis of the POSTOP clinical trial (NCT01377467) [abstract]. American Journal of Transplantation 2016;16(Suppl 3):580. [EMBASE: 611699497]
Praditpornsilpa 2014 {published data only}
-
- Praditpornsilpa K, Auswinporn S, Asawanonda P, Townamchai N, Avihingsanon Y, Eiam‐Ong S. UVB exposure increases serum calcidiol level in vitamin D deficiency kidney transplantation recipients [abstract no: B931]. Transplantation 2014;98(Suppl 1):528. [EMBASE: 71545313]
Psimenou 2002 {published data only}
-
- Psimenou E, Konstantinidou E, Giapraka N, Marinaki S, Kostakis A, Stathakis CP, et al. Randomised controlled study of calcitonin and etidronate in the treatment of osteoporosis in renal transplant patients [abstract no: 2316]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, USA. 2002. [CENTRAL: CN‐00416499]
Sanchez‐Escuredo 2015 {published data only}
-
- Sanchez‐Escuredo A, Fuster D, Rubello D, Muxi A, Ramos A, Campos F, et al. Monthly ibandronate versus weekly risedronate treatment for low bone mineral density in stable renal transplant patients. Nuclear Medicine Communications 2015;36(8):815‐8. [MEDLINE: ] - PubMed
Shahidi 2011 {published data only}
-
- Shahidi S, Kolahdooz S, Mortazavi M. Comparison between the effects of calcitriol and cholecalciferol on bone mineral density of renal transplant patients [abstract]. Journal of Isfahan Medical School 2012;30(197). [EMBASE: 373961649]
-
- Shahidi S, Kolahdose S, Mortazavi M. Comparison between the effects of calcitriol and cholecalciferol on bone mineral density of renal transplant patients [abstract]. Iranian Journal of Kidney Diseases 2011;5(Suppl 2):4. [EMBASE: 70673814]
Shahidi 2015 {published data only}
-
- Shahidi S, Ashrafi F, Mohammadi M, Moeinzadeh F, Atapour A. Low‐dose pamidronate for treatment of early bone loss following kidney transplantation: a randomized controlled trial. Iranian Journal of Kidney Diseases 2015;9(1):50‐5. [MEDLINE: ] - PubMed
Sharma 2002a {published data only}
-
- Sharma RK, Jeloka T, Gupta A, Gupta S, Gulati S, Kapoor R. Effect of alendronate on post renal transplant osteoporosis: a randomised study [abstract no: M‐PO50059]. Nephrology 2005;10(Suppl 1):A96. [CENTRAL: CN‐00782943]
-
- Sharma RK, Jeloka T, Gupta A, Gupta S, Gulati S, Kapoor R, et al. Effect of alendronate of post renal transplant osteoporosis: a randomised study [abstract no: O153]. Transplantation 2004;78(2 Suppl):60. [CENTRAL: CN‐00509474]
-
- Sharma RK, Jeloka T, Gupta A, Gupta S, Gulati S, Sharma AP. Effect of alendronate on post renal transplant osteoporosis: a randomized study [abstract no: SU‐PO544]. Journal of the American Society of Nephrology 2003;14(Nov):652A. [CENTRAL: CN‐00583910]
-
- Sharma RK, Jeloka T, Gupta A, Gupta S, Gulati S, Sharma AP, et al. Role of alendronate in post renal transplant osteoporosis: a randomised study [abstract]. Indian Journal of Nephrology 2002;12(4):236‐7. [CENTRAL: CN‐00461717]
-
- Sharma RK, Jeloka T, Gupta S, Gupta S, Gulati S, Sharma AP, et al. Role of alendronate on post renal transplant osteoporosis: a randomised study [abstract no: M775]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):249. [CENTRAL: CN‐00447697]
Sirsat 2010 {published data only}
-
- Sirsat RA, Puri VS, Holkar SD, Almeida AF, Langote AC, Kothari JP, et al. Is pamidronate more effective than alendronate in adult patients for the prevention of bone loss after kidney transplantation? [abstract no: PUB116]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):836A.
Smerud 2012 {published data only}
-
- Smerud KT, Asberg A, Kile H, Pasch A, Dahle DO, Bollerslev J, et al. A rapid and sustained improvement of calcification propensity score (serum T50) after successful kidney transplantation: reanalysis of a randomized controlled trial of ibandronate. Clinical Transplantation 2017;31(12):e13131. [MEDLINE: ] - PubMed
-
- Smerud KT, Dolgos S, Olsen IC, Asberg A, Sagedal S, Reisaeter AV, et al. A 1‐year randomized, double‐blind, placebo‐controlled study of intravenous ibandronate on bone loss following renal transplantation. American Journal of Transplantation 2012;12(12):3316‐25. [MEDLINE: ] - PubMed
-
- Smerud KT, Dolgos S, Olsen IC, Asberg A, Sagedal S, Reisaeter AV, et al. A one year prospective, randomised, double‐blind, placebo‐controlled study of intravenous ibandronate on bone loss following renal transplantation [abstract no: O‐144]. Transplant International 2011;24(Suppl 2):40. [EMBASE: 70527221]
Starke 2012 {published data only}
-
- Starke A, Corsenca A, Kohler T, Knubben J, Kraenzlin M, Uebelhart D, et al. Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(9):1461‐72. [MEDLINE: ] - PMC - PubMed
Tałałaj 1996 {published data only}
-
- Talalaj M, Gradowska L, Marcinowska‐Suchowierska E, Durlik M, Gaciong Z, Lao M. Efficiency of preventive treatment of glucocorticoid‐induced osteoporosis with 25‐hydroxyvitamin D3 and calcium in kidney transplant patients. Transplantation Proceedings 1996;28(6):3485‐7. [MEDLINE: ] - PubMed
Thervet 2008 {published data only}
-
- Thervet E, Courbebaisse M, Couberbielle JC, Zuber J, Martinez F, Mamzer MF, et al. Prospective evaluation of the risk benefit ratio of a vitamin D treatment during the early period after renal transplantation [abstract no: 697]. Transplantation 2008;86(Suppl 2):244. [CENTRAL: CN‐00783956]
Tiryaki 2015 {published data only}
-
- Tiryaki O, Usalan C, Coban S, Yildiz F. Vitamin D receptor activation with calcitriol reduce urinary angiotensinogen A marker of intrarenal renin angiotensin system correlates with albuminuria in patients with hypertensive chronic renal allograft nephropathy [abstract no: P275]. Transplant International 2015;28(Suppl 4):462. [EMBASE: 72112261]
Torregrosa 2003 {published and unpublished data}
-
- Torregrosa JV, Moreno A, Gutierrez A, Vidal S, Oppenheimer F. Alendronate for treatment of renal transplant patients with osteoporosis. Transplantation Proceedings 2003;35(4):1393‐5. [MEDLINE: ] - PubMed
Torregrosa 2007 {published data only}
-
- Torregrosa JV, Fuster D, Pedroso S, Diekmann F, Campistol JM, Rubi S, et al. Weekly risedronate in kidney transplant patients with osteopenia. Transplant International 2007;20(8):708‐11. [MEDLINE: ] - PubMed
Torregrosa 2010 {published data only}
-
- Torregrosa JV, Fuster D, Gentil MA, Marcen R, Guirado L, Zarraga S, et al. Open‐label trial: effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation 2010;89(12):1476‐81. [MEDLINE: ] - PubMed
-
- Torregrosa JV, Gentil MA, Marcen R, Zarraga S, Bravo J, Guirado L, et al. Open‐label trial: weekly risedronate immediately after transplantation in kidney recipients with osteopenia and vascular calcification [abstract no: M728]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Torregrosa 2011 {published data only}
-
- Torregrosa JV, Fuster D, Monegal A, Gentil MA, Bravo J, Guirado L, et al. Efficacy of low doses of pamidronate in osteopenic patients administered in the early post‐renal transplant. Osteoporosis International 2011;22(1):281‐7. [MEDLINE: ] - PubMed
Torres 2004 {published data only}
-
- Garcia S, Barrios Y, Gonzalez A, Concepcion MT, Checa MD, Lorenzo V, et al. Oral calcitriol prevents early bone loss after renal transplantation (RT): double blind placebo controlled study [abstract no: 3036]. Journal of the American Society of Nephrology 2000;11(Sept):575A. [CENTRAL: CN‐00601909]
-
- Torres A, Garcia S, Gomez A, Gonzalez A, Barrios Y, Concepcion MT, et al. Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney International 2004;65(2):705‐12. [MEDLINE: ] - PubMed
Trabulus 2008 {published data only}
-
- Trabulus S, Altiparmak MR, Apaydin S, Serdengecti K, Sariyar M. Treatment of renal transplant recipients with low bone mineral density: a randomized prospective trial of alendronate, alfacalcidol, and alendronate combined with alfacalcidol. Transplantation Proceedings 2008;40(1):160‐6. [MEDLINE: ] - PubMed
Trillini 2015 {published data only}
-
- Trillini M, Reyes Loaeza J, Courville K, Ferrer‐Siles C, Prandini S, Gaspari F, et al. Oral paricalcitol ameliorates post‐transplant hyperparathyroidism and proteinuria in kidney transplantation [abstract no: SA‐PO975]. Journal of the American Society of Nephrology 2013;24(Abstracts):850A.
Ugur 2000 {published data only}
-
- Uğur A, Guvener N, Isiklar I, Karakayali H, Erdal R. Efficiency of preventive treatment for osteoporosis after renal transplantation. Transplantation Proceedings 2000;32(3):556‐7. [MEDLINE: ] - PubMed
Walsh 2009 {published data only}
-
- Cunningham J, Altmann P, Pattison J, Wilkie M, Dudley C, Cockwell P, et al. Effect of pamidronate (aredia) on bone loss and fracture after renal transplantation [abstract no: TH‐PO121]. Journal of the American Society of Nephrology 2006;17(Abstracts):131A. [CENTRAL: CN‐00783004]
-
- Walsh SB, Altmann P, Pattison J, Wilkie M, Yaqoob MM, Dudley C, et al. Effect of pamidronate on bone loss after kidney transplantation: a randomized trial. American Journal of Kidney Diseases 2009;53(5):856‐65. [MEDLINE: ] - PubMed
Wissing 2005 {published data only}
-
- Wissing KM, Broeders N, Moreno‐Reyes R, Gervy C, Stallenberg B, Abramowicz D. A controlled study of vitamin D3 to prevent bone loss in renal‐transplant patients receiving low doses of steroids. Transplantation 2005;79(1):108‐15. [MEDLINE: ] - PubMed
-
- Wissing M, Broeders N, Schoutens A, Abramowicz D. A prospective and randomized study of calcium and cholecalciferol treatment to prevent bone loss after renal transplantation [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):952A. [CENTRAL: CN‐00550566]
References to studies excluded from this review
Ambuhl 1999 {published data only}
-
- Ambuhl PM, Meier D, Wolf B, Dydak U, Boesiger P, Binswanger U. Metabolic aspects of phosphate replacement therapy for hypophosphatemia after renal transplantation: impact on muscular phosphate content, mineral metabolism, and acid/base homeostasis. American Journal of Kidney Diseases 1999;34(5):875‐83. [MEDLINE: ] - PubMed
Ardalan 2007 {published data only}
-
- Ardalan MR, Maljaei H, Shoja MM, Piri AR, Khosroshahi HT, Noshad H, et al. Calcitriol started in the donor, expands the population of CD4+CD25+ T cells in renal transplant recipients. Transplantation Proceedings 2007;39(4):951‐3. [MEDLINE: ] - PubMed
Campistol 1999 {published data only}
-
- Campistol JM, Backman L, Brattstrom C, Kreis H, Morales JM, Sirolimus European Renal Transplant Study Group. Influence of immunosuppressive treatment on markers of bone remodeling in renal transplantation: comparison between cyclosporine and sirolimus [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):754A. [CENTRAL: CN–00550450]
Campistol 2000 {published data only}
-
- Campistol JM, Cole E, Gioud‐Paquet M, US European and Global Renal Transplant Study Groups. Sirolimus (rapamune): effects on bone in renal transplant patients [abstract]. Transplantation 2000;69(8 Suppl):S289. [CENTRAL: CN–00444659]
El‐Haggan 2002 {published data only}
-
- El‐Haggan W, Barthe N, Berger F, Castaing F, Chauveau P, Vendrely B, et al. Changes in bone mineral density (BMD) in kidney transplant recipients receiving tacrolimus (FK506) versus cyclosporine (Cy‐A) [abstract no: A4637]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):886A‐887A. [CENTRAL: CN‐00433625]
-
- El‐Haggan W, Barthe N, Vendrely B, Chauveau P, Berger F, Aparicio M, et al. One year evolution of bone mineral density in kidney transplant recipients receiving tacrolimus versus cyclosporine. Transplantation Proceedings 2002;34(5):1817‐8. [MEDLINE: ] - PubMed
James 2003 {published data only}
-
- James G, Raggi P, Boulay A, Burke S, Chasan‐Taber S, Chertow G, et al. Thoracic vertebral bone density increases with Renagel and decreases with calcium‐based phosphate binder therapy ‐ a two year study [abstract no: W423]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):681. [CENTRAL: CN‐00445894]
Josephson 2004 {published data only}
-
- Josephson MA, Schum LP, Chiu MY, Marshall C, Sprague SM. Post‐transplant osteopenia [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):759A. [CENTRAL: CN‐00583636]
-
- Josephson MA, Schumm LP, Chiu MY, Marshall C, Sprague SM. Calcium and calcitriol prophylaxis against post‐transplant bone loss [abstract]. Journal of the American Society of Nephrology 2000;11(Program & Abstracts):720A. [CENTRAL: CN‐00583638]
-
- Josephson MA, Schumm LP, Chiu MY, Marshall C, Thistlethwaite JR, Sprague SM. Calcium and calcitriol prophylaxis attenuates posttransplant bone loss. Transplantation 2004;78(8):1233‐6. [MEDLINE: ] - PubMed
Labib 1999 {published data only}
-
- Labib B, Ibrahim MA, Shawarby M, Abudl Rahman A, Abdel Wahab M, Elagroudy A. Effect of calculation therapy on renal osteodystrophy [abstract]. XXXVI Congress of the European Renal Association European Dialysis & Transplant Association; 1999 Sep 5‐8; Madrid, Spain. 1999:42. [CENTRAL: CN‐00484726]
Lebranchu 1999 {published data only}
-
- Hene RJ, M55002 Study Group. A randomized, double‐blind, multi‐center trial comparing two corticosteroid regimens in combination with mycophenolate mofetil (MMF) and cyslosporine (CYA) in renal transplant recipients [abstract no: 420]. Transplantation 1998;65(12):S107. [CENTRAL: CN‐00763818]
-
- Lebranchu Y. Comparison of two corticosteroid regimens in combination with CellCept and cyclosporine A for prevention of acute allograft rejection: 12 month results of a double‐blind, randomized, multi‐center study. M 55002 Study Group. Transplantation Proceedings 1999;31(1‐2):249‐50. [MEDLINE: ] - PubMed
-
- Lebranchu Y, Aubert P, Bayle F, Bedrossian J, Berthoux F, Bourbigot B, et al. Could steroids be withdrawn in renal transplant patients sequentially treated with ATG, cyclosporine, and cellcept? One‐year results of a double‐blind, randomized, multicenter study comparing normal dose versus low‐dose and withdrawal of steroids. M 55002 French Study Group. Transplantation Proceedings 2000;32(2):396‐7. [MEDLINE: ] - PubMed
-
- Lebranchu Y, M55002 Study Group. A comparison of two corticosteroid regimens in kidney transplanted patients treated with ATG/OKT3 induction, mycophenolate mofetil (MMF) and cyclosporine A (CyA) for prevention of acute allograft rejection. 12 months results of a double‐blind, randomized, multi‐center study [abstract no: 932]. Transplantation 1999;67(7):S239. [CENTRAL: CN‐00763351] - PubMed
-
- Nowacka‐Cieciura E, Durlik M, Cieciura T, Kukula K, Lewandowska D, Baczkowska T, et al. Elevated serum immunoglobulins after steroid withdrawal in renal allograft recipients. Transplantation Proceedings 2002;34(2):564‐6. [MEDLINE: ] - PubMed
Lippuner 1996 {published data only}
-
- Lippuner K, Haller B, Casez JP, Montandon A, Jaeger P. Effect of disodium monofluorophosphate, calcium and vitamin D supplementation on bone mineral density in patients chronically treated with glucocorticosteroids: a prospective, randomized, double‐blind study. Mineral & Electrolyte Metabolism 1996;22(4):207‐13. [MEDLINE: ] - PubMed
Lippuner 1998 {published data only}
-
- Lippuner K, Casez JP, Horber F, Dijkhuis E, Jaeger P. Glucocorticosteroids (GC) early after kidney transplantation (KT): beneficial effect of deflazacort (DFZ) versus prednisone (PRED) on bone and body composition in a double blind study [abstract no: A3223]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):692A. [CENTRAL: CN‐00446424]
-
- Lippuner K, Casez JP, Horber FF, Jaeger P. Effects of deflazacort versus prednisone on bone mass, body composition, and lipid profile: a randomized, double blind study in kidney transplant patients. Journal of Clinical Endocrinology & Metabolism 1998;83(11):3795‐802. [MEDLINE: ] - PubMed
Masse 2001 {published data only}
-
- Masse M, Girardin C, Ouimet D, Dandavino R, Boucher A, Madore F, et al. Initial bone loss in kidney transplant recipients: a prospective study. Transplantation Proceedings 2001;33(1‐2):1211. [MEDLINE: ] - PubMed
NCT00646282 {published data only}
-
- Raggi P, Guasch A. Mineral metabolism and vascular effects of Vitamin D therapy in kidney. www.clinicaltrials.gov/ct2/show/NCT00646282 (first received 25 March 2008).
Ponticelli 1997 {published data only}
-
- Aroldi A, Tarantino A, Montagnino G, Cesana B, Cocucci C, Ponticelli C. Effects of three immunosuppressive regimens on vertebral bone density in renal transplant recipients: a prospective study. Transplantation 1997;63(3):380‐6. [MEDLINE: ] - PubMed
-
- Goffin E, Devogelaer JP, Depresseux G, Squifflet JP, Pirson Y. Osteoporosis after organ transplantation. Lancet 2001;357(9268):1623. [MEDLINE: ] - PubMed
-
- Montagnino G, Tarantino A, Maccario M, Elli A, Cesana B, Ponticelli C. Long‐term results with cyclosporine monotherapy in renal transplant patients: a multivariate analysis of risk factors. American Journal of Kidney Diseases 2000;35(6):1135‐43. [MEDLINE: ] - PubMed
-
- Montagnino G, Tarantino A, Segoloni GP, Cambi V, Rizzo G, Altieri P, et al. Long‐term results of a randomized study comparing three immunosuppressive schedules with cyclosporine in cadaveric kidney transplantation. Journal of the American Society of Nephrology 2001;12(10):2163‐9. [MEDLINE: ] - PubMed
-
- Ponticelli C. Steroid withdrawal in organ transplant recipients [abstract no: 0351]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00583616]
Reed 2004 {published data only}
-
- Reed J, Keightley G, Blumenthal S, Bradley C, LaBrecque J, Turner S, et al. The CONTROL STUDY: enhanced achievement of NKF‐K/DOQITM bone metabolism and disease targets using cinacalcet HCI (SensiparTM) [abstract no: F‐PO986]. Journal of the American Society of Nephrology 2004;15(Program & Abstracts):280–1A. [CENTRAL: CN–00550527]
Rigotti 2003 {published data only}
-
- Rigotti P, Vialtel P, Pascual J, Sanchez‐Fructuoso A, Escuin F, the Bone Mineral Density Study Group. Immunosuppression without maintenance steroids prevents decline of bone mineral density following renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):199. [CENTRAL: CN–00447406]
ter Meulen 2003 {published data only}
-
- Hendrikx TK, Klepper M, Ijzermans J, Weimar W, Baan CC. Clinical rejection and persistent immune regulation in kidney transplant patients. Transplant Immunology 2009;21(3):129‐35. [MEDLINE: ] - PubMed
-
- Hesselink DA, Ngyuen H, Wabbijn M, Smak Gregoor PJ, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids [abstract no: 1290]. American Journal of Transplantation 2003;3(Suppl 5):482. - PMC - PubMed
-
- Ter Meulen CG, Riemsdijk IC, Hene RJ, Christiaans MHL, Gelder T, Hilbrands LB, et al. A prospective randomized trial comparing steroid‐free immunosuppresion with limited steroid exposure on bone mineral density in the first year after renal transplantation [abstract no: 0344]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00402832]
-
- ter Meulen CG, Goertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, et al. Decreased renal excretion of soluble interleukin‐2 receptor alpha after treatment with daclizumab. Kidney International 2003;64(2):697‐703. [MEDLINE: ] - PubMed
THOMAS 2002 {published data only}
-
- Boots JM, Ham EC, Christiaans MH, Hooff JP. Risk of adrenal insufficiency with steroid maintenance therapy in renal transplantation. Transplantation Proceedings 2002;34(5):1696‐7. [MEDLINE: ] - PubMed
-
- Budde K, Salmela K, Pascual J, Rigotti P, THOMAS Study Group. Steroid‐withdrawal in tacrolimus‐treated renal transplant recipients: results of a 3‐year follow‐up study [abstract no: 221]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550470]
-
- Jindal RM, Salmela K, Vanrentergheim Y, Hooff JP, Squifflet JP. Reduction of high cholesterol levels by early withdrawal of steroids from a tacrolimus‐based triple regimen [abstract no: 206]. American Journal of Transplantation 2002;2(Suppl 3):190. [CENTRAL: CN‐00520347]
-
- Pascual J, Hooff JP, Salmela K, Budde K, Rigotti P, Lang P. Long‐term efficacy and safety of steroid‐withdrawal in tacrolimus treated renal transplant recipients: results of a 3 year follow‐up [abstract no: 1524]. American Journal of Transplantation 2004;4(Suppl 8):576‐7. [CENTRAL: CN‐00615852]
-
- Pascual J, Hooff JP, Salmela K, Lang P, Rigotti P, Budde K. Three‐year observational follow‐up of a multicenter, randomized trial on tacrolimus‐based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation 2006;82(1):55‐61. [MEDLINE: ] - PubMed
Vasquez 2004 {published data only}
-
- Vasquez EM, Sifontis N, Jacobssen V, Testa G, Sankary H, Benedetti E. Simvastatin for prevention of bone loss following renal transplantation [abstract no: P284]. Transplantation 2004;78(2 Suppl):293. [CENTRAL: CN‐00509537]
Zaoui 2003 {published data only}
-
- Zaoui P, Vialtel P, Rigotti P, Pascual J, Sanchez‐Fructuoso A, Escuin F, et al. A steroid‐free immunosuppressive regimen of daclizumab, tacrolimus and MMF prevents loss of bone mass following renal transplantation [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):495. [CENTRAL: CN–00448519]
References to studies awaiting assessment
Jorge 2016 {published data only}
-
- Jorge C, Adragao T, Matias P, Bruges M, Birne R, Laranjinha I, et al. A prospective randomized controlled study of cholecalciferol supplementation in kidney transplant recipients‐ preliminary results at 30 months [abstract no: TH‐PO766]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):271A.
Marques 2019 {published data only}
-
- Marques I, Araujo MJC, Graciolli F, dos Reis L, Pereira RM, Custodio M, et al. A prospective and randomized trial of zoledronic acid to prevent bone loss in the first year after kidney transplantation. [abstract no: TH‐PO923]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):339. - PMC - PubMed
NCT01675089 {published data only}
-
- David‐Neto E. Zoledronic acid to prevent bone loss after kidney transplantation. www.clinicaltrials.gov/ct2/show/NCT01675089 (first received 23 August 2012).
Oblak 2017 {published data only}
-
- Arnol M, Oblak M, Mlinsek G, Bren AF, Buturovic‐Ponikvar J, Kandus A. Proteinuria in kidney transplant recipients: Prevalence in a national cohort and study design of the effect of paricalcitol for reduction of proteinuria [abstract no: RO‐204]. Transplant International 2011;24(Suppl 2):185. [EMBASE: 70527733]
-
- Arnol M, Oblak M, Mlinsek G, Buturovic‐Ponikvar J, Kandus A. Paricalcitol versus placebo for reduction of proteinuria in kidney transplant recipients: a double‐blind, randomized controlled trial [abstract]. Transplantation 2016;100(7 Suppl 1):S214‐5. [EMBASE: 613005120] - PubMed
-
- Oblak M, Mlinsek G, Kandus A, Buturovic‐Ponikvar J, Arnol M. Effects of paricalcitol on biomarkers of inflammation and fibrosis in kidney transplant recipients: results of a randomized controlled trial. Clinical Nephrology 2017;88(13):119‐25. [MEDLINE: ] - PubMed
-
- Oblak M, Mlinsek G, Kandus A, Buturovic‐Ponikvar J, Arnol M. Paricalcitol versus placebo for reduction of proteinuria in kidney transplant recipients: a double‐blind, randomized controlled trial. Transplant International 2018;31(12):1391‐404. [MEDLINE: ] - PubMed
Tiryaki 2018 {published data only}
-
- Tiryaki O, Usalan C, Tarakcioglu M, Coban S. Calcitriol reduces albuminuria and urinary angiotensinogen level in renal transplant recipients. Transplantation Proceedings 2018;50(5):1342‐7. [MEDLINE: ] - PubMed
References to ongoing studies
NCT00748618 {published data only}
-
- Larsen J. Vitamin D replacement after kidney transplant. www.clinicaltrials.gov/ct2/show/NCT00748618 (first received 4 September 2008).
NCT00889629 {published data only}
-
- Markell M. Pilot study: Effects of hectorol (doxercalciferol) vitamin D replacement on proteinuria, PTH level and bone turnover in stable kidney transplant recipients: a single‐blind, placebo‐controlled study in patients receiving 25‐oh vitamin D3. www.clinicaltrials.gov/ct2/show/NCT00889629 (first received 23 April 2009).
NCT02224144 {published data only}
-
- Nickolas T. A comparative trial of calcitriol versus placebo for the preservation of bone mass and strength after kidney transplantation. www.clinicaltrials.gov/ct2/show/NCT02224144 (first received 22 August 2014).
VITA‐D 2009 {published data only}
-
- Thiem U, Gessl A, Heinze G, Gossler U, Perkmann T, Kainberger F, et al. Influence of high‐dose vitamin D3 therapy on calcitriol levels in vitamin‐D‐deficient kidney transplant recipients: preliminary results of a randomized controlled trial [abstract]. Austrian Journal of Clinical Endocrinology & Metabolism 2011;4:19. [EMBASE: 70805209]
-
- Thiem U, Gessl A, Heinze G, Gossler U, Perkmann T, Kainberger F, et al. Treatment of vitamin D deficiency and its impact on albumin excretion in kidney transplant recipients: preliminary results of a randomized controlled trial [abstract no: P‐303]. Transplant International 2011;24(Suppl 2):303. [EMBASE: 70528199]
-
- Thiem U, Heinze G, Gossler U, Perkmann T, Winkler S, Marculescu R, et al. Vitamin D for improving the outcome after kidney transplantation: rationale, design, and baseline characteristics of the participants of the VITA‐D randomized controlled trial [abstract no: P520]. Transplant International 2013;26(Suppl 2):289. [EMBASE: 71360130]
-
- Thiem U, Heinze G, Segel R, Perkmann T, Kainberger F, Muhlbacher F, et al. VITA‐D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: a randomized, placebo‐controlled study to evaluate the post‐transplant outcome. Trials [Electronic Resource] 2009;10:36. [MEDLINE: ] - PMC - PubMed
-
- Thiem U, Heinze G, Segel R, Perkmann T, Kainberger F, Muhlbacher F, et al. Vita‐D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: preliminary report of a RCT to evaluate the post‐transplant outcome [abstract no: Sa673]. NDT Plus 2010;3(Suppl 3):iii269. [EMBASE: 70484139] - PMC - PubMed
VITALE 2014 {published data only}
-
- Courbebaisse M, Alberti C, Colas S, Prie D, Souberbielle JC, Treluyer JM, et al. VITamin D supplementation in renAL transplant recipients (VITALE): a prospective, multicentre, double‐blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double‐blind, randomized, controlled trial. Trials [Electronic Resource] 2014;15(1):430. [MEDLINE: ] - PMC - PubMed
Additional references
Abbott 2001
-
- Abbott KC, Oglesby RJ, Hypolite IO, Kirk AD, Ko CW, Welch PG, et al. Hospitalizations for fractures after renal transplantation in the United States. Annals of Epidemiology 2001;11(7):450‐7. [MEDLINE: ] - PubMed
Adachi 1997
-
- Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, et al. Intermittent etidronate therapy to prevent corticosteroid induced osteoporosis. New England Journal of Medicine 1997;337(6):382‐7. [MEDLINE: ] - PubMed
Almond 1994
-
- Almond MK, Kwan JT, Evans K, Cunningham J. Loss of regional bone mineral density in the first 12 months following renal transplantation. Nephron 1994;66(1):52‐7. [MEDLINE: ] - PubMed
Baker 2010
-
- Baker R, Jardine A, MacTier R. How do the KDIGO Clinical Practice Guidelines on the care of kidney transplant recipients apply to the UK?. www.renal.org/wp‐content/uploads/2017/06/management‐of‐the‐kidney‐transp... (accessed 12 June 2019).
Baker 2017
-
- Baker RJ, Mark PB, Patel RK, Stevens KK, Palmer N. Renal Association Clinical Practice Guideline. Post‐operative care in the kidney transplant recipient. www.renal.org/wp‐content/uploads/2017/06/final‐post‐operative‐care‐guide... (accessed 12 June 2019). - PMC - PubMed
Brown 2009
-
- Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, Gregorio LH, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. Journal of Bone & Mineral Research 2009;24(1):153‐61. [MEDLINE: ] - PubMed
Chadban 2009
-
- Chadban S, Chan M, Fry K, Patwardhan A, Ryan C, Trevillian P, et al. Nutritional interventions for the prevention of bone disease in kidney transplant recipients. Nephrology 2010;15 Suppl 1:S43‐7. [MEDLINE: ] - PubMed
Chambers 1980
-
- Chambers TJ. Diphosphonates inhibit bone resorption by macrophages in vitro. Journal of Pathology 1980;132(3):255‐62. [MEDLINE: ] - PubMed
Chesnut 2000
-
- Chesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. American Journal of Medicine 2000;109(4):267‐76. [MEDLINE: ] - PubMed
Chonchol 2009
-
- Chonchol M, Locatelli F, Abboud HE, Charytan C, Francisco AL, Jolly S, et al. A randomized, double‐blind, placebo‐controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. American Journal of Kidney Diseases 2009;53(2):197‐207. [MEDLINE: ] - PubMed
Cummings 2009
-
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New England Journal of Medicine 2009;361(8):756‐65. [MEDLINE: ] - PubMed
De Nijs 2004
-
- Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW. Prevention and treatment of glucocorticoid‐induced osteoporosis with active vitamin D3 analogues: a review with meta‐analysis of randomized controlled trials including organ transplantation studies. Osteoporosis International 2004;15(8):589‐602. [MEDLINE: ] - PubMed
de Vries 1982
Durieux 2002
-
- Durieux S, Mercadal L, Orcel, P, Dao H, Rioux C, Bernard M, et al. Bone mineral density and fracture prevalence in long‐term kidney graft recipients. Transplantation 2002;74(4):496‐500. [MEDLINE: ] - PubMed
ERBP 2011
-
- Heemann U, Abramowicz D, Spasovski G, VanHolder R, European Renal Best Practice (ERBP) Work Group on Kidney Transplantation. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines on kidney transplantation: a European Renal Best Practice (ERBP) position statement. Nephrology Dialysis and Transplantation 2011;26(7):2099‐106. [MEDLINE: ] - PubMed
Fryer 1996
-
- Fryer J, Grant D, Jiang J, Metrakos P, Ozcay N, Ford C, et al. Influence of macrophage depletion on bacterial translocation and rejection in small bowel transplantation. Transplantation 1996;62(5):553‐8. [MEDLINE: ] - PubMed
GRADE 2008
Grey 1994
-
- Grey AB, Cundy TF, Reid IR. Continuous combined oestrogen/progestin therapy is well tolerated and increases bone density at the hip and spine in post‐menopausal osteoporosis. Clinical Endocrinology 1994;40(5):671‐7. [MEDLINE: ] - PubMed
Grotz 1994
-
- Grotz WH, Mundinger FA, Gugel B, Exner V, Kirste G, Schollmeyer PJ. Bone fracture and osteodensitometry with dual energy X‐ray absorptiometry in kidney transplant recipients. Transplantation 1994;58(8):912‐5. [MEDLINE: ] - PubMed
Grotz 1995
-
- Grotz WH, Mundinger FA, Rasenack J, Speidel L, Olschewski M, Exner VM, et al. Bone loss after kidney transplantation: a longitudinal study in 115 graft recipients. Nephrology Dialysis Transplantation 1995;10(11):2096‐100. [MEDLINE: ] - PubMed
Hahn 1981
-
- Hahn TJ, Halstead LR, Baran DT. Effects of short term glucocorticoid administration on intestinal calcium absorption and circulating vitamin D metabolite concentrations in man. Journal of Clinical Endocrinology & Metabolism 1981;52(1):111‐5. [MEDLINE: ] - PubMed
Hahn 2015
Hariharan 2001
-
- Hariharan S. Long‐term kidney transplant survival. American Journal of Kidney Diseases 2001;38(6 Suppl 6):S44‐50. [MEDLINE: ] - PubMed
Heaf 2000
-
- Heaf J, Tvedegaard E, Kanstrup IL, Fogh‐Andersen N. Bone loss after renal transplantation: role of hyperparathyroidism, acidosis, cyclosporine and systemic disease. Clinical Transplantation 2000;14(5):457‐63. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horber 1994
-
- Horber FF, Casez JP, Steiger U, Czerniak A, Montandon A, Jaeger P. Changes in bone mass early after kidney transplantation. Journal of Bone & Mineral Research 1994;9(1):1‐9. [MEDLINE: ] - PubMed
Hung 1996
-
- Hung CJ, Lee PC, Song CM, Chang YT, Tsai MT, Dai YS, et al. Clinical implication of hormone treatment in postmenopausal kidney transplants. Transplantation Proceedings 1996;28(3):1548‐50. [MEDLINE: ] - PubMed
Jeffrey 2003
-
- Jeffrey JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation 2003;76(10):1498‐502. [MEDLINE: ] - PubMed
Julian 1991
-
- Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. New England Journal of Medicine 1991;325(8):544‐50. [MEDLINE: ] - PubMed
Karachalios 2004
-
- Karachalios T, Lyritis GP, Kaloudis J, Roidis N, Katsiri M. The effects of calcitonin on acute bone loss after pertrochanteric fractures. Journal of Bone & Joint Surgery ‐ British Volume 2004;86(3):350‐8. [MEDLINE: ] - PubMed
KDIGO CKD‐MBD Guideline 2009
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD‐MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD). Kidney International ‐ Supplement 2009, (113):S1‐130. [MEDLINE: ] - PubMed
KDIGO Transplant Guideline 2009
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐155. [MEDLINE: ] - PubMed
KDOQI 2010
-
- Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, et al. KDOQI US Commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD‐Mineral and Bone Disorder (CKD‐MBD). American Journal of Kidney Diseases 2010;55(5):773‐99. [MEDLINE: ] - PubMed
Knoll 2010
-
- Knoll GA, Blydt‐Hansen TD, Campbell P, Cantarovich M, Cole E, Fairhead T, et al. Canadian Society of Transplantation and Canadian Society of Nephrology commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Kidney Diseases 2010;56(2):219‐46. [MEDLINE: ] - PubMed
Krol 2014a
-
- Krol CG, Dekkers OM, Kroon HM, Rabelink TJ, Koek B, Hamdy NA. No association between BMD and prevalent vertebral fractures in liver transplant recipients at time of screening for transplantation. Journal of Clinical Endocrinology & Metabolism 2014;99(10):3677‐85. [MEDLINE: ] - PubMed
Krol 2014b
-
- Krol CG, Dekkers OM, Kroon HM, Rabelink TJ, Hoek B, Hamdy NA. Longitudinal changes in BMD and fracture risk in orthotopic liver transplant recipients not using bone‐modifying treatment. Journal of Bone & Mineral Research 2014;29(8):1763‐9. [MEDLINE: ] - PubMed
Malluche 2010
Monier‐Faugere 2000
-
- Monier‐Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. Journal of the American Society of Nephrology 2000;11(6):1093‐9. [MEDLINE: ] - PubMed
Neer 2001
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1‐34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine 2001;344(19):1434‐41. [MEDLINE: ] - PubMed
Nisbeth 1999
-
- Nisbeth U, Lindh E, Ljunghall S, Backman U, Fellstrom B. Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation 1999;67(9):1218‐22. [MEDLINE: ] - PubMed
O'Shaunessy 2002
-
- O'Shaughnessy EA, Dahl DC, Smith CL, Kasiske BL. Risk factors for fractures in kidney transplantation. Transplantation 2002;74(3):362‐6. [MEDLINE: ] - PubMed
Orwoll 2003
-
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez‐Perez A, et al. The effect of teriparatide [human parathyroid hormone (1‐34)] therapy on bone density in men with osteoporosis. Journal of Bone & Mineral Research 2003;18(1):9‐17. [MEDLINE: ] - PubMed
Pichette 1996
-
- Pichette V, Bonnardeaux A, Prudhomme L, Gagne M, Cardinal J, Ouimet D. Long‐term bone loss in kidney transplant recipients: a cross‐sectional and longitudinal study. American Journal of Kidney Diseases 1996;28(1):105‐14. [MEDLINE: ] - PubMed
Raggi 2004
-
- Raggi P, Burke S, Chasen‐Taber S, Chertow G, Holzer H, Bommer J. Sevelamer preserves trabecular bone mineral density [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:103‐4. [CENTRAL: CN‐00509430]
Reid 1996
-
- Reid IR, Wattie DJ, Evans MC, Stapleton JP. Testosterone therapy in glucocorticoid‐treated men. Archives of Internal Medicine 1996;156(11):1173‐7. [MEDLINE: ] - PubMed
Sambrook 1988
-
- Sambrook PN, Eisman JA, Champion GD, Pocock NA. Sex hormone status and osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis & Rheumatism 1988;31(8):973‐8. [MEDLINE: ] - PubMed
Schunemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sprague 2004
-
- Sprague SM, Josephson MA. Bone disease after kidney transplantation. Seminars in Nephrology 2004;24(1):82‐90. [MEDLINE: ] - PubMed
Torres 2002
-
- Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. Journal of the American Society of Nephrology 2002;13(2):551‐8. [MEDLINE: ] - PubMed
Vautour 2004
-
- Vautour LM, Melton LJ 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long‐term fracture risk following renal transplantation: a population based study. Osteoporosis International 2004;15(2):160‐7. [MEDLINE: ] - PubMed
Veenstra 1999
-
- Veenstra DL, Best JH, Hornberger J, Sullivan SD, Hricik DE. Incidence and long‐term cost of steroid‐related side effects after renal transplantation. American Journal of Kidney Diseases 1999;33(5):829‐39. [MEDLINE: ] - PubMed
Versele 2016
-
- Versele EB, Laecke S, Dhondt AW, Verbeke F, Vanholder R, Biesen W, et al. Bisphosphonates for preventing bone disease in kidney transplant recipients: a meta‐analysis of randomized controlled trials. Transplant International 2016;29(2):153‐64. [MEDLINE: ] - PubMed
Wang 2016
-
- Wang J, Yao M, Xu JH, Shu B, Wang YJ, Cui XJ. Bisphosphonates for prevention of osteopenia in kidney‐transplant recipients: a systematic review of randomized controlled trials. Osteoporosis International 2016;27(5):1683‐90. [MEDLINE: ] - PubMed
Weisinger 2006
-
- Weisinger JR, Carlini RG, Rojas E, Bellorin‐Font E. Bone disease after renal transplantation. Clinical Journal of The American Society of Nephrology: CJASN 2006;1(6):1300‐13. [MEDLINE: ] - PubMed
References to other published versions of this review
Palmer 2004
Palmer 2005a
Palmer 2005b
-
- Palmer SC, Strippoli GF, McGregor DO. Interventions for preventing bone disease in kidney transplant recipients: a systematic review of randomized controlled trials. American Journal of Kidney Diseases 2005;45(4):638‐49. [MEDLINE: ] - PubMed
Publication types
LinkOut - more resources
Full Text Sources

