Systemic GDF11 stimulates the secretion of adiponectin and induces a calorie restriction-like phenotype in aged mice
- PMID: 31637864
- PMCID: PMC6974718
- DOI: 10.1111/acel.13038
Systemic GDF11 stimulates the secretion of adiponectin and induces a calorie restriction-like phenotype in aged mice
Abstract
Aging is a negative regulator of general homeostasis, tissue function, and regeneration. Changes in organismal energy levels and physiology, through systemic manipulations such as calorie restriction and young blood infusion, can regenerate tissue activity and increase lifespan in aged mice. However, whether these two systemic manipulations could be linked has never been investigated. Here, we report that systemic GDF11 triggers a calorie restriction-like phenotype without affecting appetite or GDF15 levels in the blood, restores the insulin/IGF-1 signaling pathway, and stimulates adiponectin secretion from white adipose tissue by direct action on adipocytes, while repairing neurogenesis in the aged brain. These findings suggest that GDF11 has a pleiotropic effect on an organismal level and that it could be a linking mechanism of rejuvenation between heterochronic parabiosis and calorie restriction. As such, GDF11 could be considered as an important therapeutic candidate for age-related neurodegenerative and metabolic disorders.
Keywords: GDF11; adiponectin; aging; calorie restriction; heterochronic parabiosis; rejuvenation.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


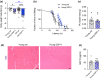
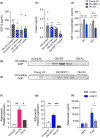
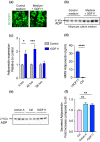
References
-
- Berryman, D. E. , List, E. O. , Palmer, A. J. , Chung, M. Y. , Wright‐Piekarski, J. , Lubbers, E. , … Kopchick, J. J. (2010). Two‐year body composition analyses of long‐lived GHR null mice. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 65, 31–40. 10.1093/gerona/glp175 - DOI - PMC - PubMed
-
- Bonkowski, M. S. , Rocha, J. S. , Masternak, M. M. , Al Regaiey, K. A. , & Bartke, A. (2006). Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proceedings of the National Academy of Sciences of the United States of America, 103, 7901–7905. 10.1073/pnas.0600161103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

