Ammonium tetrathiomolybdate enhances the antitumor effect of cisplatin via the suppression of ATPase copper transporting beta in head and neck squamous cell carcinoma
- PMID: 31638244
- PMCID: PMC6826331
- DOI: 10.3892/or.2019.7367
Ammonium tetrathiomolybdate enhances the antitumor effect of cisplatin via the suppression of ATPase copper transporting beta in head and neck squamous cell carcinoma
Abstract
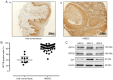
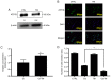
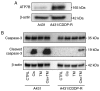
Platinum‑based antitumor agents have been widely used to treat head and neck squamous cell carcinoma (HNSCC) and numerous other malignancies. Cisplatin is the most frequently used platinum‑based antitumor agent, however drug resistance and numerous undesirable side effects limit its clinical efficacy for cancer patients. Cancer cells discharge cisplatin into the extracellular space via copper transporters such as ATPase copper transporting beta (ATP7B) in order to escape from cisplatin‑induced cell death. In the present study, it was demonstrated for the first time that the copper chelator ammonium tetrathiomolybdate (TM) has several promising effects on cisplatin and HNSCC. First, TM suppressed the ATP7B expression in HNSCC cell lines in vitro, thereby enhancing the accumulation and apoptotic effect of cisplatin in the cancer cells. Next, it was revealed that TM enhanced the antitumor effect of cisplatin in HNSCC cell tumor progression in a mouse model of bone invasion, which is important since HNSCC cells frequently invade to facial bone. Finally, it was demonstrated that TM was able to overcome the cisplatin resistance of a human cancer cell line, A431, via ATP7B depression in vitro.
Figures




References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et al. Global, Regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A Systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

