Mobile phone text messaging and app-based interventions for smoking cessation
- PMID: 31638271
- PMCID: PMC6804292
- DOI: 10.1002/14651858.CD006611.pub5
Mobile phone text messaging and app-based interventions for smoking cessation
Abstract
Background: Mobile phone-based smoking cessation support (mCessation) offers the opportunity to provide behavioural support to those who cannot or do not want face-to-face support. In addition, mCessation can be automated and therefore provided affordably even in resource-poor settings. This is an update of a Cochrane Review first published in 2006, and previously updated in 2009 and 2012.
Objectives: To determine whether mobile phone-based smoking cessation interventions increase smoking cessation rates in people who smoke.
Search methods: For this update, we searched the Cochrane Tobacco Addiction Group's Specialised Register, along with clinicaltrials.gov and the ICTRP. The date of the most recent searches was 29 October 2018.
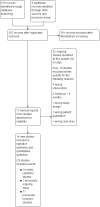
Selection criteria: Participants were smokers of any age. Eligible interventions were those testing any type of predominantly mobile phone-based programme (such as text messages (or smartphone app) for smoking cessation. We included randomised controlled trials with smoking cessation outcomes reported at at least six-month follow-up.
Data collection and analysis: We used standard methodological procedures described in the Cochrane Handbook for Systematic Reviews of Interventions. We performed both study eligibility checks and data extraction in duplicate. We performed meta-analyses of the most stringent measures of abstinence at six months' follow-up or longer, using a Mantel-Haenszel random-effects method, pooling studies with similar interventions and similar comparators to calculate risk ratios (RR) and their corresponding 95% confidence intervals (CI). We conducted analyses including all randomised (with dropouts counted as still smoking) and complete cases only.
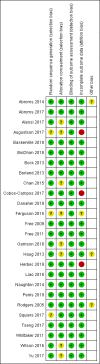
Main results: This review includes 26 studies (33,849 participants). Overall, we judged 13 studies to be at low risk of bias, three at high risk, and the remainder at unclear risk. Settings and recruitment procedures varied across studies, but most studies were conducted in high-income countries. There was moderate-certainty evidence, limited by inconsistency, that automated text messaging interventions were more effective than minimal smoking cessation support (RR 1.54, 95% CI 1.19 to 2.00; I2 = 71%; 13 studies, 14,133 participants). There was also moderate-certainty evidence, limited by imprecision, that text messaging added to other smoking cessation interventions was more effective than the other smoking cessation interventions alone (RR 1.59, 95% CI 1.09 to 2.33; I2 = 0%, 4 studies, 997 participants). Two studies comparing text messaging with other smoking cessation interventions, and three studies comparing high- and low-intensity messaging, did not show significant differences between groups (RR 0.92 95% CI 0.61 to 1.40; I2 = 27%; 2 studies, 2238 participants; and RR 1.00, 95% CI 0.95 to 1.06; I2 = 0%, 3 studies, 12,985 participants, respectively) but confidence intervals were wide in the former comparison. Five studies compared a smoking cessation smartphone app with lower-intensity smoking cessation support (either a lower-intensity app or non-app minimal support). We pooled the evidence and deemed it to be of very low certainty due to inconsistency and serious imprecision. It provided no evidence that smartphone apps improved the likelihood of smoking cessation (RR 1.00, 95% CI 0.66 to 1.52; I2 = 59%; 5 studies, 3079 participants). Other smartphone apps tested differed from the apps included in the analysis, as two used contingency management and one combined text messaging with an app, and so we did not pool them. Using complete case data as opposed to using data from all participants randomised did not substantially alter the findings.
Authors' conclusions: There is moderate-certainty evidence that automated text message-based smoking cessation interventions result in greater quit rates than minimal smoking cessation support. There is moderate-certainty evidence of the benefit of text messaging interventions in addition to other smoking cessation support in comparison with that smoking cessation support alone. The evidence comparing smartphone apps with less intensive support was of very low certainty, and more randomised controlled trials are needed to test these interventions.
Conflict of interest statement
RW was co‐author of one paper on one of the included studies (Rodgers 2005). She was a co‐investigator on included studies (Baskerville 2018; Free 2009; Free 2011), and principle investigator of a further included study (Whittaker 2011). RW's institution (Auckland UniServices Ltd) received grant money to cover the costs of providing the text messaging intervention for the study described in Free 2011. RW's institution licensed the STOMP text messaging cessation intervention in 2008, however no royalties were received. The licence has since been rescinded. This is not deemed to be a conflict of interest.
HM was co‐author of Whittaker 2011 and received honoraria from Pfizer for speaking at educational events and attending advisory group meetings.
CB was co‐author of Whittaker 2011 and his institution received grant money to cover the costs of providing the text messaging intervention for the study described in Free 2011.
AR was a lead author (Rodgers 2005), and a co‐author (Free 2009; Free 2011; Whittaker 2011), on included studies.
YG none known.
RD's institution received grant money to cover the costs of providing the text messaging intervention for the study described in Free 2011.
Figures














Comment in
-
Review: Automated mobile phone text messaging increases smoking cessation (moderate evidence).Ann Intern Med. 2020 Mar 17;172(6):JC33. doi: 10.7326/ACPJ202003170-033. Ann Intern Med. 2020. PMID: 32176899 No abstract available.
References
References to studies included in this review
Abroms 2014 {published data only}
Abroms 2017 {published data only}
Alessi 2017 {published data only}
Augustson 2017 {published data only}
Baskerville 2018 {published data only}
-
- Baskerville N, Struik L, Hammond D, Guindon G, Norman C, Whittaker R, et al. Effect of a mobile phone intervention on quitting smoking in a young adult population of smokers: randomized controlled trial study protocol. JMIR Research Protocols 2015;4(1):e10. [DOI: 10.2196/resprot.3823] - DOI - PMC - PubMed
-
- NCT01983150. A randomized controlled trial to test the effect of a smartphone quit smoking intervention on young adult smokers. clinicaltrials.gov/ct2/show/NCT01983150 (first received 13 November 2013).
BinDhim 2018 {published data only}
-
- ACTRN12613000833763. Assessing the effect of an interactive decision‐aid smartphone smoking cessation application (app) on quit rates: a double‐blind randomized control trial [In adult smokers, does an interactive smartphone smoking cessation application, compared to standard information, increase the quit rates?]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364613 (first received 17 July 2013).
Bock 2013 {published data only}
Borland 2013 {published and unpublished data}
-
- Balmford J, Borland R, Benda P, Howard S. Factors associated with use of automated smoking cessation interventions: findings from the eQuit study. Health Education Research 2013;28(2):288‐99. [CENTRAL: 921007; CRS: 9400107000001543; PUBMED: 23107931] - PubMed
-
- Borland R, Balmford J, Benda P. Population‐level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction 2013;108(3):618‐28. [CENTRAL: 921001; CRS: 9400107000000767; PUBMED: 22994457] - PubMed
Chan 2015 {published data only}
-
- Chan SS, Wong DC, Cheung YT, Leung DY, Lau L, Lai V, et al. A block randomized controlled trial of a brief smoking cessation counselling and advice through short message service on participants who joined the Quit to Win Contest in Hong Kong. Health Education Research 2015;30(4):609‐21. [DOI: 10.1093/her/cyv023] - DOI - PMC - PubMed
Cobos‐Campos 2017 {published data only}
Danaher 2019 {published data only}
-
- NCT01952236. Web and mobile smoking cessation (MobileQuit). clinicaltrials.gov/show/nct01952236 (first received 27 September 2013).
Ferguson 2015 {published and unpublished data}
-
- Ferguson SG, Walters JA. The effect of mobile phone text messages on short and long term quitting in motivated smokers: a randomised controlled trial. Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28; Philadelphia. 2015. [CRS: 9400131000001072; POS3‐80]
-
- Schuz N, Walters JA, Frandsen M, Bower J, Ferguson SG. Compliance with an EMA monitoring protocol and its relationship with participant and smoking characteristics. Nicotine & Tobacco Research 2014;16(S2):S88‐92. [CRS: 9400129000001511; EMBASE: 2014262309] - PubMed
Free 2009 {published and unpublished data}
-
- Free C, Whittaker R, Knight R, Abramsky T, Rodgers A, Roberts IG. Txt2stop: a pilot randomised controlled trial of mobile phone‐based smoking cessation support. Tobacco Control 2009;18:88‐91. - PubMed
Free 2011 {published and unpublished data}
-
- Free C, Hoile E, Robertson S, Knight R. Three controlled trials of interventions to increase recruitment to a randomized controlled trial of mobile phone based smoking cessation support. Clinical Trials 2010;7(3):265‐73. - PubMed
-
- Michie S, Free C, West R. Characterising the 'Txt2Stop' smoking cessation text messaging intervention in terms of behaviour change techniques. Journal of Smoking Cessation 2012;7(1):55‐60. [CRS: 9400123000018452]
Garrison 2018 {published data only}
Haug 2013 {published and unpublished data}
-
- Haug S, Meyer C, Dymalski A, Lippke S, John U. Efficacy of a text messaging (SMS) based smoking cessation intervention for adolescents and young adults: study protocol of a cluster randomised controlled trial. BMC Public Health 2012;12:51. [CENTRAL: 814353; CRS: 9400123000011961; EMBASE: 22260736; PUBMED: 22260736] - PMC - PubMed
-
- Haug S, Schaub MP, Venzin V, Meyer C, John U. Moderators of outcome in a text messaging (SMS)‐based smoking cessation intervention for young people [Differenzielle Wirksamkeit eines Short Message Service (SMS)‐basierten Programms zur Forderung des Rauchstopps bei Jugendlichen]. Psychiatrische Praxis 2013;40(6):339‐46. [CENTRAL: 875575; CRS: 9400126000000210; EMBASE: 2013576012; PUBMED: 24008683] - PubMed
-
- Haug S, Schaub Michael P, Schmid H. Predictors of adolescent smoking cessation and smoking reduction. Patient Education and Counseling 2014;95(3):378‐83. [CRS: 9400129000001807; PUBMED: 24674150] - PubMed
Herbec 2019 {unpublished data only}
-
- Herbec A, Shahab L, Matei A, Brown J, Kaur Ubhi H, et al. Does inclusion of craving management tools increase effectiveness and usage of a stop smoking app? Results from BupaQuit trial. 3rd UCL Centre for Behaviour Change Digital Health Conference 2017: Harnessing digital technology for behaviour change; 2017 Feb 22‐23; London, UK. 2017.
-
- ISRCTN10548241. Study of effectiveness of a smartphone application for quitting smoking. isrctn.com/ISRCTN10548241 (first received 10 February 2015).
Liao 2018 {published data only}
Naughton 2014 {published data only}
-
- Naughton F, Jamison J, Boase S, Sloan M, Gilbert H, Prevost AT, et al. Randomized controlled trial to assess the short‐term effectiveness of tailored web‐ and text‐based facilitation of smoking cessation in primary care (iQuit in Practice). Addiction 2014;109(7):1184‐93. [CENTRAL: 1051137; CRS: 9400129000001642; PUBMED: 24661312] - PMC - PubMed
-
- Sutton S, Smith S, Jamison J, Boase S, Mason D, Prevost AT, et al. Study protocol for iQuit in Practice: a randomised controlled trial to assess the feasibility, acceptability and effectiveness of tailored web‐ and text‐based facilitation of smoking cessation in primary care. BMC Public Health 2013;13:324. [CENTRAL: 873088; CRS: 9400126000000015; PUBMED: 23575031] - PMC - PubMed
Peiris 2019 {published data only}
Rodgers 2005 {published data only}
-
- Bramley D, Riddell T, Whittaker R, Corbett T, Lin R‐B, Wills M. Smoking cessation using mobile phone text messaging is as effective in Maori as non‐Maori. New Zealand Medical Journal 2005;118(1216):1494‐504. - PubMed
Squiers 2017 {published data only}
-
- Squiers L, Augustson E, Brown D, Kelly B, Southwell B, Dever J, et al. An experimental comparison of mobile texting programs to help young adults quit smoking. Health Systems 2017;6:1‐14.
Tseng 2017 {published and unpublished data}
-
- NCT01898195. Improving adherence to smoking cessation medication among PLWHA (HIV). clinicaltrials.gov/show/NCT01898195 (first received 12 July 2013). [CRS: 9400129000001221]
-
- Shelley D, Krebs P, Schoenthaler A, Urbina A, Gonzalez M, Tseng T‐Y, et al. Correlates of adherence to varenicline among HIV+ smokers: a path analysis. Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28; Philadelphia. 2015. [CRS: 9400131000001079; PA14‐2] - PMC - PubMed
-
- Tseng TY, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, et al. Combining text messaging and telephone counselling to increase varenicline adherence and smoking abstinence among cigarette smokers living with HIV: a randomized controlled trial. AIDS Behavior 2017;21(7):1964‐74. - PMC - PubMed
Whittaker 2011 {published and unpublished data}
Wilson 2016 {published data only}
-
- NCT01723163. Abstinence Reinforcement Therapy (ART) for rural veteran smokers. www.clinicaltrials.gov/ct2/show/NCT01723163 (first received 7th November 2012).
Yu 2017 {published data only}
-
- ChiCTR‐OIC‐17010803. Changchun Smoke‐Free Home mHealth Program [Creating smoke‐free homes for infants and increasing quitting among fathers through education and mHealth interventions in Changchun, China]. chictr.org.cn/showprojen.aspx?proj=18143 (first received 06 March 2017).
References to studies excluded from this review
ACTRN12617000491369 {published data only}
-
- ACTRN12617000491369. Quittr: a game that wants smokers to quit [Quittr: a smoking cessation serious game. Determining if game features influence smoker engagement and retention during a quit attempt]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372661 (first received 29 March 2017).
Aigner 2017 {published data only}
Applegate 2007 {published data only}
-
- Applegate B, Raymond C, Collado‐Rodriguez A, Riley W, Schneider N. Improving adherence to nicotine gum by SMS text messaging: a pilot study. Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 Feb 21‐24; Austin, Texas. 2007. [RPOS3‐57]
Bamidis 2017 {published data only}
-
- Bamidis P, Paraskevopoulos E, Konstantinidis E, Spachos D, Billis A. Multimodal e‐health services for smoking cessation and public health: the SmokeFreeBrain project approach. Studies in Health Technology and Informatics 2017;245:5‐9. - PubMed
Bernstein 2018 {published data only}
Blasco 2012 {published data only}
-
- Blasco A, Carmona M, Fernandez‐Lozano I, Salvador CH, Pascual M, Sagredo PG, et al. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(1):25‐31. [CENTRAL: 814645; CRS: 9400123000012404; 6831; PUBMED: 22113368] - PubMed
Brendryen 2008 {published data only}
-
- Brendryen H, Kraft P. Happy Ending: a randomized controlled trial of a digital multi‐media smoking cessation intervention. Addiction 2008;103:478‐84. [doi:10.1111/j.1360‐0443.2007.02119.x] - PubMed
Bricker 2014 {published data only}
-
- Bricker J, Mull K, Kientz J, Vilardaga R, Mercer L, Akioka K, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug and Alcohol Dependence 2014;143(1):87‐94. [CENTRAL: 1047686; CRS: 9400050000000151; EMBASE: 2014721228; PUBMED: 25085225] - PMC - PubMed
Brinker 2016 {published data only}
-
- Brinker T, Holzapfel J, Baudson T, Sies K, Jakob L, Baumert H, et al. Photoaging smartphone app promoting poster campaign to reduce smoking prevalence in secondary schools: the Smokerface randomized trial: design and baseline characteristics. BMJ Open 2016;6(11):e014288. [DOI: 10.1136/bmjopen-2016-014288] - DOI - PMC - PubMed
Bronshtein 2016 {published data only}
-
- Bronshtein E, Ondersma S, Sokol R. Optimizing ehealth for smoking in pregnancy: a pilot factorial evaluation of providing single vs multiple options. Reproductive Sciences 2016;S1:195A.
Buller 2014 {published data only}
Chow 2012 {published data only}
-
- Chow CK, Redfern J, Thiagalingam A, Jan S, Whittaker R, Hackett M, et al. Design and rationale of the tobacco, exercise and diet messages (TEXT ME) trial of a text message‐based intervention for ongoing prevention of cardiovascular disease in people with coronary disease: a randomised controlled trial protocol. BMJ Open 2012;2(1):e000606. - PMC - PubMed
Dale 2014 {published data only}
-
- Dale L, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self‐management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials 2014;15:71. [CENTRAL: 984614; CRS: 9400129000001546; EMBASE: 2014230967; PUBMED: 24588893] - PMC - PubMed
Fingrut 2014 {published data only}
Fraser 2014 {published data only}
Gritz 2013 {published data only}
-
- Gritz E, Vidrine D, Marks R, Arduino R. A randomized trial of an innovative cell phone intervention for smokers living with HIV/AIDS. Society for Research on Nicotine & Tobacco 17th Annual Meeting; 2011 Feb 16‐19; Toronto, Canada. 2011. [SYM 10A]
-
- NCT00502827. Smoking Cessation for HIV/AIDS Patients. clinicaltrials.gov/ct2/show/NCT00502827 (first received 26 February 2016).
Halpern 2018 {published data only}
-
- Halpern S, Harhay M, Saulsgiver K, Brophy C, Troxel A, Volpp K. A pragmatic trial of e‐cigarettes, incentives, and drugs for smoking cessation. New England Journal of Medicine 2018;378(24):2302‐10. - PubMed
-
- NCT02328794. Randomized clinical trial to reduce harm from tobacco. clinicaltrials.gov/ct2/show/NCT02328794 (first received 31 December 2014).
Hammett 2018 {published data only}
-
- Hammett E, Veldheer S, Hrabovsky S, Yingst J, Berg A, Poole E, et al. TXT2STAYQUIT: pilot randomized trial of brief automated smoking cessation texting intervention for inpatient smokers discharged from the hospital. Journal of Hospital Medicine 2018;13(7):488‐9. [DOI: 10.12788/jhm.2907] - DOI - PMC - PubMed
Hassandra 2017 {published data only}
-
- Hassandra M, Lintunen T, Hagger MS, Heikkinen R, Vanhala M, Kettunen T. An mHealth App for supporting quitters to manage cigarette cravings with short bouts of physical activity: a randomized pilot feasibility and acceptability study. JMIR Mhealth and Uhealth 2017;5(5):e74. [DOI: 10.2196/mhealth.6252] - DOI - PMC - PubMed
Haug 2008 {published data only}
-
- Haug S, Meyer C, Gross B, Schorr G, Thyrian JR, Kordy H, et al. Continuous individual support of smoking cessation in socially deprived young adults via mobile phones ‐ results of a pilot study. Gesundheitswesen 2008;70(6):364‐71. - PubMed
Haug 2009 {published data only}
-
- Haug S, Meyer C, John U. Individual support of smoking cessation using text messaging: results of two pilot studies. Sucht 2008;54(5):316. [CENTRAL: 794115; CRS: 9400123000006096]
-
- Haug S, Meyer C, Schorr G, Bauer S, John U. Continuous individual support of smoking cessation using text messaging: a pilot experimental study. Nicotine & Tobacco Research 2009;11(8):915‐23. - PubMed
Haug 2014 {published data only}
-
- Haug S, Paz Castro R, Filler A, Kowatsch T, Fleisch E, Schaub MP. Efficacy of an internet and SMS‐based integrated smoking cessation and alcohol intervention for smoking cessation in young people: study protocol of a two‐arm cluster randomised controlled trial. BMC Public Health 2014;14:1140. [CENTRAL: 1053487; CRS: 9400129000003374] - PMC - PubMed
Kiselev 2011 {published data only}
-
- Kiselev AR, Shvarts VA, Posnenkova OM, Gridnev VI, Dovgalevskii P, Oshchepkova EV, et al. Outpatient prophylaxis and treatment of arterial hypertension with application of mobile telephone systems and Internet techniques. Terapevticheskiĭ Arkhiv 2011;83(4):46‐52. - PubMed
Lazev 2004 {published data only}
-
- Lazev A, Vidrine D, Arduino R, Gritz E. Increasing access to smoking cessation treatment in a low‐income, HIV‐positive population: the feasibility of using cellular telephones. Nicotine & Tobacco Research 2004;6(2):281‐6. [doi: 10.1080/4622200410001676314] - PubMed
Mason 2016 {published data only}
Mehring 2014 {published data only}
Naughton 2012 {published data only}
-
- Naughton F, Prevost A, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self‐help intervention for pregnant smokers (MiQuit). Nicotine & Tobacco Research 2012;14(5):569‐77. - PubMed
Naughton 2017 {published data only}
-
- Naughton F, Cooper S, Foster K, Emery J, Leonardi‐Bee J, Sutton S, et al. Large multi‐centre pilot randomized controlled trial testing a low‐cost, tailored, self‐help smoking cessation text message intervention for pregnant smokers (MiQuit). Addiction 2017;112(7):1238‐49. [DOI: 10.1111/add.13802] - DOI - PMC - PubMed
NCT01454999 {unpublished data only}
-
- Jordan P. Tailored texting to enhance a stage‐based online tailored program for veterans who smoke: a randomized breakthrough and benchmark trial [personal communication]. Email 2015.
-
- NCT01454999. Cell phone‐based expert systems for smoking cessation (TXT). clinicaltrials.gov/ct2/show/NCT01454999 (first received 19 October 2011).
NCT02245308 {published data only}
-
- NCT02245308. Abstinence reinforcement therapy (ART) for homeless veteran smokers. clinicaltrials.gov/show/NCT02245308 (first received 19 September 2014). [CRS: 9400131000001050]
NCT02844296 {published data only}
-
- NCT02844296. Short‐bout handgrip exercise for smoking cessation (SHESC). clinicaltrials.gov/ct2/show/NCT02844296 (first received 26 July 2016).
NCT03177265 {published data only}
-
- NCT03177265. Text messaging to engage and retain veterans in smoking cessation counseling (TiMES). clinicaltrials.gov/ct2/show/NCT03177265 (first received 6 June 2017).
Obermayer 2004 {published data only}
-
- Obermayer JL, Riley WT, Asif O, Jean‐Mary J. College smoking cessation using cell phone text messaging. Journal of American College Health 2004;53(2):71‐8. - PubMed
Pechmann 2015 {published data only}
-
- Pechmann C, Pan L, Delucchi K, Lakon CM, Prochaska JJ. Development of a Twitter‐based intervention for smoking cessation that encourages high‐quality social media interactions via automessages. Journal of Medical Internet Research 2015;17(2):e50. [CRS: 9400131000001029; PUBMED: 25707037] - PMC - PubMed
-
- Prochaska JJ, Pechmann C, Lakon C, Delucchi K. Randomized controlled trial of tweet2quit for smoking cessation. Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28; Philadelphia. 2015. [CRS: 9400131000001026; PA21‐2]
Peng 2013 {published data only}
-
- Peng WB, Schoech D. Evaluation of a web‐phone intervention system in changing smoking behavior ‐ a randomized controlled trial. Journal of Technology in Human Services 2013;31(3):248‐68. [CENTRAL: 1053486; CRS: 9400129000000455]
Pollak 2013 {published data only}
Riley 2008 {published data only}
-
- Riley W, Obermayer J, Jean‐Mary J. Internet and mobile phone text messaging intervention for college smokers. Journal of American College Health 2008;57(2):245‐8. - PubMed
Shi 2013 {published data only}
-
- Shi HJ, Jiang XX, Yu CY, Zhang Y. Use of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescents. Journal of Telemedicine and Telecare 2013;19(5):282‐7. [CENTRAL: 984362; CRS: 9400126000000380; PUBMED: 24163238] - PubMed
Skov‐Ettrup 2013 {published data only}
-
- Skov‐Ettrup LS, Dalum P, Ekholm O, Tolstrup JS. Reach and uptake of Internet‐ and phone‐based smoking cessation interventions: results from a randomized controlled trial. Preventive Medicine 2014;62:38‐43. [CENTRAL: 1001289; CRS: 9400129000000774; EMBASE: 2014140700] - PubMed
-
- Skov‐Ettrup LS, Dalum P, Tolstrup JS. Internet‐based intervention and telephone counselling for smoking cessation: results from a 4‐arm randomised controlled trial. European Journal of Epidemiology 2013;28(S1):S48‐9. [CENTRAL: 1011884; CRS: 9400130000000339; EMBASE: 71300831]
Skov‐Ettrup 2014 {published data only}
-
- Skov‐Ettrup LS, Ringgaard LW, Dalum P, Flensborg‐Madsen T, Thygesen LC, Tolstrup JS. Comparing tailored and untailored text messages for smoking cessation: a randomized controlled trial among adolescent and young adult smokers. Health Education Research 2014;29(2):195‐205. [CRS: 9400129000003462; PUBMED: 24399268] - PubMed
Skov‐Ettrup 2016 {published data only}
Snider 2011 {published data only}
-
- Snider J. Cell phone text messaging may boost smoking quit rates. Journal of the American Dental Association 2011;142(8):901‐2. - PubMed
Stanczyk 2014 {published data only}
-
- Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. Influence of delivery strategy on message‐processing mechanisms and future adherence to a Dutch computer‐tailored smoking cessation intervention. Journal of Medical Internet Research 2013;15(2):e28. [CENTRAL: 873085; CRS: 9400107000001436; PUBMED: 23388554] - PMC - PubMed
Vidrine 2006 {published data only}
-
- Vidrine D, Arduino R, Gritz E. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine & Tobacco Research 2006;8(S1):S103‐8. - PubMed
-
- Vidrine D, Arduino R, Lazev A, Gritz E. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 2006;20:253‐60. [ISSN 0269‐9370] - PubMed
Vilaplana 2014 {published data only}
-
- Vilaplana J, Solsona F, Abella F, Cuadrado J, Alves R, Mateo J. S‐PC: an e‐treatment application for management of smoke‐quitting patients. Computer Methods and Programs in Biomedicine 2014;115(1):33‐45. [CENTRAL: 988111; CRS: 9400050000000047; EMBASE: 2014296941; PUBMED: 24742965] - PubMed
Wizner 2009 {published data only}
-
- Wizner B, Gaciong Z, Narkiewicz K, Grodzicki T. Education using SMS increases efficacy of treatment of hypertensive patients [Zwiększenie skuteczności terapii hipotensyjnej u pacjentów z nadciśnieniem tętniczym dzięki edukacji przez SMS]. Nadcisnienie Tetnicze 2009;13(3):147‐57.
Ybarra 2012 {published and unpublished data}
-
- Ybarra ML, Holtrop JS, Bosi AT, Bilir N, Korchmaros JD, Emri AK. Feasibility and acceptability of a text messaging‐based smoking cessation program in Ankara, Turkey. Journal of Health Communication 2013;18(8):960‐73. [CRS: 9400126000000575; PUBMED: 23627304] - PubMed
Ybarra 2013 {published and unpublished data}
-
- Ybarra ML, Holtrop JS, Prescott TL, Strong D. Process evaluation of a mHealth program: lessons learned from Stop my Smoking USA, a text messaging‐based smoking cessation program for young adults. Patient Education and Counseling 2014;97(2):236‐43. [CRS: 9400131000000800; PUBMED: 25103183] - PMC - PubMed
Yuhongxia 2011 {published data only}
-
- Yuhongxia L. The compliance of varenicline usage and the smoking abstinence rate via mobile phone text messaging combine with varenicline: a single‐blind, randomised control trial. Respirology 2011;16 :46‐7.
References to ongoing studies
Cambon 2017 {published data only}
Collins 2017 {published data only}
CTRI201801011643 {published data only}
-
- CTRI/2018/01/011643. Tobacco cessation at non communicable disease clinics [Development of tobacco cessation training package and assessing its impact on tobacco use behavior of patients attending non communicable diseases clinics of Punjab]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=20950&EncHid=&userN... (first received 31 January 2018).
CTRI201803012401 {published data only}
-
- CTRI/2018/03/012401. A clinical trial to study the effect of WhatsApp and pamphlet based quit smoking interference among software professionals in Bengaluru City [Effectiveness of WhatsApp based smoking cessation intervention among software professional in Bengaluru City: a randomised controlled trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=21448&EncHid=&u... (first received 07 March 2018).
Graham 2016 {published data only}
Graham 2017 {published data only}
ISRCTN11154315 {published data only}
-
- ISRCTN11154315. Efficacy of a smoking cessation intervention using smartphones. isrctn.com/ISRCTN11154315 (first received 04 April 2018).
ISRCTN11318024 {published data only}
-
- ISRCTN11318024. Impact of a smartphone application on smoking cessation: a randomized controlled trial. isrctn.com/ISRCTN11318024 (first received 26 April 2018).
ISRCTN15396225 {published data only}
-
- ISRCTN15396225. Evaluation of the effectiveness of a text‐based mHealth smoking cessation intervention among high school students in Sweden. isrctn.com/ISRCTN15396225 (first received 10 October 2017).
ISRCTN16022919 {published data only}
-
- ISRCTN16022919. Mobile health interventions for smoking cessation services uptake and smoking cessation: a factorial randomised trial in Thailand. isrctn.com/ISRCTN16022919 (first received 11 November 2016).
ISRCTN17964518 {published data only}
-
- ISRCTN17964518. Evaluation of the "Stop Tabac" Android phone application. isrctn.com/ISRCTN17964518 (first received 24 June 2015).
ISRCTN33869008 {published data only}
-
- ISRCTN33869008. Mobile phone‐based smoking cessation intervention for patients with elective surgery. isrctn.com/ISRCTN33869008 (first received 17 Auguts 2018).
NCT01982110 {published data only}
-
- NCT01982110. A mindfulness based application for smoking cessation. clinicaltrials.gov/show/NCT01982110 (first received 13 November 2013). [CRS: 9400131000001042]
NCT01990079 {published data only}
-
- NCT01990079. Use of technological advances to prevent smoking relapse among smokers with PTSD. clinicaltrials.gov/show/NCT01990079 (first received 21 November 2013). [CRS: 9400131000001036]
NCT01995097 {published data only}
-
- NCT01995097. BABY STEPS II: SMS scheduled gradual reduction text messages to help pregnant smokers quit. clinicaltrials.gov/ct2/show/NCT01995097 (first received 26 November 2013).
NCT02037360 {published data only}
-
- NCT02037360. Mobile mindfulness training for smoking cessation. clinicaltrials.gov/show/NCT02037360 (first received 15 January 2014). [CRS: 9400131000001048]
NCT02218281 {published data only}
-
- NCT02218281. Developing a smartphone app with mindfulness training for teen smoking cessation. clinicaltrials.gov/show/NCT02218281 (first received 15 January 2014). [CRS: 9400131000001087]
NCT02218944 {published data only}
-
- NCT02218944. Response inhibition training in smoking cessation. clinicaltrials.gov/ct2/show/NCT02037360 (first received 15 January 2014).
NCT02237898 {published data only}
-
- NCT02237898. Harnessing the power of technology: MOMBA for postpartum smoking. clinicaltrials.gov/show/NCT02237898 (first received 11 September 2014). [CRS: 9400131000001085]
NCT02420015 {published data only}
-
- NCT02420015. Mobile health technology to enhance abstinence in smokers with schizophrenia. clinicaltrials.gov/ct2/show/NCT02420015 (first received 17 April 2015).
NCT02665208 {published data only}
-
- NCT02665208. A pilot text messaging intervention to reduce smoking in office‐based buprenorphine and inpatient detoxification patients. clinicaltrials.gov/ct2/show/NCT02665208 (first received 27 January 2016).
NCT02724462 {published data only}
-
- NCT02724462. Trial of an innovative smartphone intervention for smoking cessation. clinicaltrials.gov/ct2/show/NCT02665208 (first received 27 January 2016).
NCT02840513 {published data only}
-
- NCT02840513. Smartphone app and CO self‐monitoring for smoking cessation. clinicaltrials.gov/ct2/show/NCT02840513 (first received 21 July 2016).
NCT02901171 {published data only}
-
- NCT02901171. The contribution of a smartphone application to acceptance and commitment therapy group treatment for smoking cessation. clinicaltrials.gov/show/NCT02901171 (first received 21 July 2016).
NCT03021655 {published data only}
-
- NCT03021655. A pilot randomized control trial to help youth smokers to quit smoking. clinicaltrials.gov/ct2/show/NCT03021655 (first received 16 January 2017).
NCT03038542 {published data only}
-
- NCT03038542. Quit4hlth: enhancing tobacco and cancer control through framed text messages. clinicaltrials.gov/ct2/show/NCT03021655 (first received 16 January 2017).
NCT03191019 {published data only}
-
- NCT03191019. A mobile‐phone based intervention to support smoking cessation among Chilean women. clinicaltrials.gov/ct2/show/NCT03191019 (first received 19 June 2017).
NCT03445507 {published data only}
-
- NCT03445507. Effectiveness of a chat bot for smoking cessation: a pragmatic trial in primary care. clinicaltrials.gov/show/NCT03445507 (first received 19 June 2017).
NCT03495622 {published data only}
-
- NCT03495622. Effectiveness of a combined CHW and text messaging‐based tobacco intervention in India. clinicaltrials.gov/show/NCT03495622 (first received 12 April 2018).
NCT03538938 {published data only}
-
- NCT03538938. Improving quitline support study (IQS). clinicaltrials.gov/show/NCT03538938 (first received 28 May 2018).
NCT03552978 {published data only}
-
- NCT03552978. Tech and telephone smoking cessation treatment for young veterans with PTSD. clinicaltrials.gov/show/NCT03552978 (first received 12 June 2018).
NCT03553173 {published data only}
-
- NCT03553173. So‐Lo‐Mo intervention applied to the smoking cessation process. clinicaltrials.gov/ct2/show/NCT03553173 (first received 12 June 2018).
Valdivieso‐Lopez 2013 {published data only}
Weng 2018 {published data only}
Additional references
Abroms 2011
Armanasco 2017
-
- Armanasco AA, Miller YD, Fjeldsoe BS, Marshall AL. Preventive health behavior change text message interventions: a meta‐analysis. American Journal of Preventive Medicine 2017;52(3):391‐402. - PubMed
Boland 2017
-
- Boland V, Mattick R, McRobbie H, Siahpush M, Courtney R. “I’m not strong enough; I’m not good enough. I can’t do this, I’m failing”: a qualitative study of low‐socioeconomic status smokers’ experiences with accessing cessation support and the role for alternative technology‐based support. International Journal for Equity in Health 2017;16:196. - PMC - PubMed
Byambasuren 2018
Covidence [Computer program]
-
- Veritas Health Innovation. Covidence. Version accessed 29 October 2018. Melbourne, Australia: Veritas Health Innovation.
Dirieto 2016
-
- Direito A, Carraça E, Rawstorn J, Whittaker R, Maddison R. mHealth technologies to influence physical activity and sedentary behaviors: behavior change techniques, systematic review and meta‐analysis of randomized controlled trials. Annals of Behavioral Medicine 2016;51(2):226‐39. - PubMed
Guerriero 2013
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
IHME 2018
-
- Institute for Health Metrics and Evaluation. Findings from the Global Burden of Disease Study 2017. Seattle, WA: IHME, 2018.
ITU 2018
-
- International Telecommunications Union. Measuring the Information Society Report 2018 ‐ Volume 1. www.itu.int/en/ITU‐D/Statistics/Documents/publications/misr2018/MISR‐201... (accessed 29 March 2019).
Lunde 2018
Schoeppe 2016
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Scott‐Sheldon 2016
Thakkar 2016
-
- Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta‐analysis. JAMA Internal Medicine 2016;176(3):340‐9. - PubMed
References to other published versions of this review
Whittaker 2009
Whittaker 2012
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

