Fracture repair requires TrkA signaling by skeletal sensory nerves
- PMID: 31638597
- PMCID: PMC6877307
- DOI: 10.1172/JCI128428
Fracture repair requires TrkA signaling by skeletal sensory nerves
Abstract
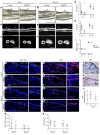
Bone is richly innervated by nerve growth factor-responsive (NGF-responsive) tropomyosin receptor kinase A-expressing (TrKa-expressing) sensory nerve fibers, which are required for osteochondral progenitor expansion during mammalian skeletal development. Aside from pain sensation, little is known regarding the role of sensory innervation in bone repair. Here, we characterized the reinnervation of tissue following experimental ulnar stress fracture and assessed the impact of loss of TrkA signaling in this process. Sequential histological data obtained in reporter mice subjected to fracture demonstrated a marked upregulation of NGF expression in periosteal stromal progenitors and fracture-associated macrophages. Sprouting and arborization of CGRP+TrkA+ sensory nerve fibers within the reactive periosteum in NGF-enriched cellular domains were evident at time points preceding periosteal vascularization, ossification, and mineralization. Temporal inhibition of TrkA catalytic activity by administration of 1NMPP1 to TrkAF592A mice significantly reduced the numbers of sensory fibers, blunted revascularization, and delayed ossification of the fracture callus. We observed similar deficiencies in nerve regrowth and fracture healing in a mouse model of peripheral neuropathy induced by paclitaxel treatment. Together, our studies demonstrate an essential role of TrkA signaling for stress fracture repair and implicate skeletal sensory nerves as an important upstream mediator of this repair process.
Keywords: Bone Biology; Bone disease.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

