Beta-band oscillations play an essential role in motor-auditory interactions
- PMID: 31639252
- PMCID: PMC7268072
- DOI: 10.1002/hbm.24830
Beta-band oscillations play an essential role in motor-auditory interactions
Abstract
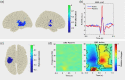
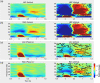
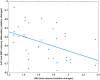
In the human brain, self-generated auditory stimuli elicit smaller cortical responses compared to externally generated sounds. This sensory attenuation is thought to result from predictions about the sensory consequences of self-generated actions that rely on motor commands. Previous research has implicated brain oscillations in this process. However, the specific role of these oscillations in motor-auditory interactions during sensory attenuation is still unclear. In this study, we aimed at addressing this question by using magnetoencephalography (MEG). We recorded MEG in 20 healthy participants during listening to passively presented and self-generated tones. Our results show that the magnitude of sensory attenuation in bilateral auditory areas is significantly correlated with the modulation of beta-band (15-30 Hz) amplitude in the motor cortex. Moreover, we observed a significant directional coupling (Granger causality) in beta-band originating from the motor cortex toward bilateral auditory areas. Our findings indicate that beta-band oscillations play an important role in mediating top-down interactions between motor and auditory cortex and, in our paradigm, suppress cortical responses to predicted sensory input.
Keywords: MEG; auditory perception; beta-band oscillation; motor-auditory interactions; prediction; sensory attenuation.
© 2019 The Authors. Human Brain Mapping published by Wiley Periodicals, Inc.
Figures

References
-
- Abbasi, O. , Dammers, J. , Arrubla, J. , Warbrick, T. , Butz, M. , Neuner, I. , & Shah, N. J. (2015). Time‐frequency analysis of resting state and evoked EEG data recorded at higher magnetic fields up to 9.4 T. Journal of Neuroscience Methods, 255, 1–11. 10.1016/j.jneumeth.2015.07.011 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

