Distinguishing Drug from Disease by Use of the Hydrashift 2/4 Daratumumab Assay
- PMID: 31639760
- PMCID: PMC7484995
- DOI: 10.1373/jalm.2018.026476
Distinguishing Drug from Disease by Use of the Hydrashift 2/4 Daratumumab Assay
Abstract
Background: Daratumumab, a monoclonal antibody used to treat relapsed or refractory multiple myeloma, can interfere with protein electrophoresis and immunofixation assays. False-positive immunofixation results due to daratumumab can cause uncertainty regarding the status of a patient's disease and lead to potential misclassification of their response to therapy. The Hydrashift 2/4 Daratumumab assay (Sebia) was recently cleared by the Food and Drug Administration for resolving daratumumab interference on immunofixation. Here, we evaluate the performance of the Hydrashift assay in multiple myeloma patients receiving treatment with daratumumab-based regimens.
Methods: Waste serum samples from multiple myeloma patients (n = 40) receiving daratumumab were analyzed by standard immunofixation and the Hydrashift assay. Results from these tests were compared and were evaluated along with pretreatment serum protein electrophoresis and immunofixation results, if available.
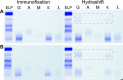
Results: The Hydrashift assay shifted the migration of daratumumab in patient samples. In 27 cases, the patient's M protein was distinguishable from daratumumab by standard immunofixation. In these cases, the Hydrashift assay confirmed that the IgGκ band was daratumumab and helped identify the presence of treatment-related oligoclonal bands. There were 11 instances in which the patient's IgGκ M protein comigrated with daratumumab. In all 11 cases, the Hydrashift assay confirmed the presence of residual M protein. Finally, in 2 patients whose pretreatment immunofixation results were not available, the Hydrashift assay confirmed that the IgGκ band visible on immunofixation was due to daratumumab alone.
Conclusions: The Hydrashift 2/4 Daratumumab assay is a useful tool to clarify the source of an IgGκ band on immunofixation and allow a patient's M protein to be viewed without interference.
© 2018 American Association for Clinical Chemistry.
Figures

References
-
- McCudden CR, Jacobs JFM, Keren D, Caillon H, Dejoie T, Andersen K. Recognition and management of common, rare, and novel serum protein electrophoresis and immunofixation interferences. Clin Biochem 2018;51:72–9. - PubMed
-
- Cenaj O, Dahlin JL, Buencamino DM, Laubach JP, Jarolim P. 74-Year-old female with new monoclonal protein on serum immunofixation electrophoresis. Clin Biochem 2018;51:97–100. - PubMed
-
- Willrich MAV, Ladwig PM, Andreguetto BD, Barnidge DR, Murray DL, Katzmann JA, et al. Monoclonal antibody therapeutics as potential interferences on protein electrophoresis and immunofixation. Clin Chem Lab Med 2016;54:1085–93. - PubMed
-
- Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

