Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress
- PMID: 31640102
- PMCID: PMC6918908
- DOI: 10.3233/JAD-190817
Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress
Abstract
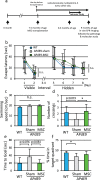
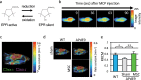
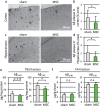
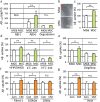
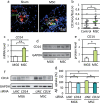
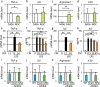
Mesenchymal stem cells (MSC) are increasingly being studied as a source of cell therapy for neurodegenerative diseases, and several groups have reported their beneficial effects on Alzheimer's disease (AD). In this study using AD model mice (APdE9), we found that transplantation of MSC via the tail vein improved spatial memory in the Morris water maze test. Using electron paramagnetic resonance imaging to evaluate the in vivo redox state of the brain, we found that MSC transplantation suppressed oxidative stress in AD model mice. To elucidate how MSC treatment ameliorates oxidative stress, we focused on amyloid-β (Aβ) pathology and microglial function. MSC transplantation reduced Aβ deposition in the cortex and hippocampus. Transplantation of MSC also decreased Iba1-positive area in the cortex and reduced activated ameboid shaped microglia. On the other hand, MSC transplantation accelerated accumulation of microglia around Aβ deposits and prompted microglial Aβ uptake and clearance as shown by higher frequency of Aβ-containing microglia. MSC transplantation also increased CD14-positive microglia in vivo, which play a critical role in Aβ uptake. To confirm the effects of MSC on microglia, we co-cultured the mouse microglial cell line MG6 with MSC. Co-culture with MSC enhanced Aβ uptake by MG6 cells accompanied by upregulation of CD14 expression. Additionally, co-culture of MG6 cells with MSC induced microglial phenotype switching from M1 to M2 and suppressed production of proinflammatory cytokines. These data indicate that MSC treatment has the potential to ameliorate oxidative stress through modification of microglial functions, thereby improving Aβ pathology in AD model mice.
Keywords: Alzheimer’s disease; CD14; electron paramagnetic resonance imaging; mesenchymal stem cells; microglia; oxidative stress.
Conflict of interest statement
Authors’ disclosures available online (
Figures

References
-
- Querfurth HW, Laferla FM (2010) Alzheimer’s disease. N Engl J Med 362, 329–344. - PubMed
-
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. - PubMed
-
- Canevari L, Abramov AY, Duchen MR (2004) Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res 29, 637–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

