Targeting tauopathy with engineered tau-degrading intrabodies
- PMID: 31640765
- PMCID: PMC6805661
- DOI: 10.1186/s13024-019-0340-6
Targeting tauopathy with engineered tau-degrading intrabodies
Abstract
Background: The accumulation of pathological tau is the main component of neurofibrillary tangles and other tau aggregates in several neurodegenerative diseases, referred to as tauopathies. Recently, immunotherapeutic approaches targeting tau have been demonstrated to be beneficial in decreasing tauopathy in animal models. We previously found that passive immunotherapy with anti-tau antibody to human tau or expression of an anti-tau secreted single-chain variable fragment (scFv) in the central nervous system of a mouse model of tauopathy decreased but did not remove all tau-associated pathology. Although these and other studies demonstrate that conventional immunotherapeutic approaches targeting tau can influence tau pathogenesis, the majority of pathological tau remains in the cytosol of cells, not typically accessible to an extracellular antibody. Therefore, we reasoned targeting intracellular tau might be more efficacious in preventing or decreasing tauopathy.
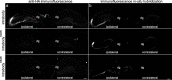
Methods: By utilizing our anti-tau scFv, we generated anti-tau intrabodies for the expression in the cytosol of neurons. To enhance the degradation capacity of conventional intrabodies, we engineered chimeric anti-tau intrabodies fused to ubiquitin harboring distinct mutations that shuttle intracellular tau for either the proteasome or lysosomal mediated degradation. To evaluate the efficacy in delaying or eliminating tauopathy, we expressed our tau degrading intrabodies or controls in human tau transgenic mice by adeno-associated virus prior to overt tau pathology and after tau deposition.
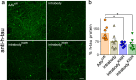
Results: Our results demonstrate, the expression of chimeric anti-tau intrabodies significantly reduce tau protein levels in primary neuronal cultures expression human tau relative to a non-modified anti-tau intrabody. We found the expression of engineered tau-degrading intrabodies destined for proteasomal-mediated degradation are more effective in delaying or eliminating tauopathy than a conventional intrabody in aged human tau transgenic mice.
Conclusion: This study, harnesses the strength of intrabodies that are amendable for targeting specific domains or modifications with the cell-intrinsic mechanisms that regulate protein degradation providing a new immunotherapeutic approach with potentially improved efficacy.
Keywords: Alzheimer’s disease; Immunotherapy; Intrabodies; Tau degradation; Tauopathy.
Conflict of interest statement
GG, HJ, CL, and DMH are listed as inventors on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies. DMH co-founded and is on the scientific advisory board of C2N Diagnostics, LLC. C2N Diagnostics, LLC has licensed certain anti-tau antibodies to AbbVie for therapeutic development. DMH is on the scientific advisory board of Denali and Genentech and consults for Idorsia.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

