Development of effective anti-influenza drugs: congeners and conjugates - a review
- PMID: 31640786
- PMCID: PMC6806523
- DOI: 10.1186/s12929-019-0567-0
Development of effective anti-influenza drugs: congeners and conjugates - a review
Abstract
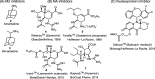
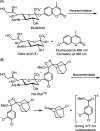
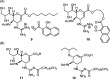
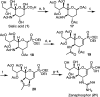
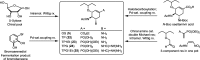
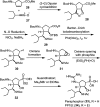
Influenza is a long-standing health problem. For treatment of seasonal flu and possible pandemic infections, there is a need to develop new anti-influenza drugs that have good bioavailability against a broad spectrum of influenza viruses, including the resistant strains. Relenza™ (zanamivir), Tamiflu™ (the phosphate salt of oseltamivir), Inavir™ (laninamivir octanoate) and Rapivab™ (peramivir) are four anti-influenza drugs targeting the viral neuraminidases (NAs). However, some problems of these drugs should be resolved, such as oral availability, drug resistance and the induced cytokine storm. Two possible strategies have been applied to tackle these problems by devising congeners and conjugates. In this review, congeners are the related compounds having comparable chemical structures and biological functions, whereas conjugate refers to a compound having two bioactive entities joined by a covalent bond. The rational design of NA inhibitors is based on the mechanism of the enzymatic hydrolysis of the sialic acid (Neu5Ac)-terminated glycoprotein. To improve binding affinity and lipophilicity of the existing NA inhibitors, several methods are utilized, including conversion of carboxylic acid to ester prodrug, conversion of guanidine to acylguanidine, substitution of carboxylic acid with bioisostere, and modification of glycerol side chain. Alternatively, conjugating NA inhibitors with other therapeutic entity provides a synergistic anti-influenza activity; for example, to kill the existing viruses and suppress the cytokines caused by cross-species infection.
Keywords: Congener; Conjugate; Drug; Influenza; Inhibitor; Neuraminidase.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









References
-
- Syrjänen RK, Jokinen J, Ziegler T, Sundman J, Lahdenkari M, Julkunen I, Kilpi TM. Effectiveness of pandemic and seasonal influenza vaccines in preventing laboratory-confirmed influenza in adults: a clinical cohort study during epidemic seasons 2009-2010 and 2010-2011 in Finland. PLoS One. 2014;9(9):e108538. doi: 10.1371/journal.pone.0108538. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

