Evaluation of multiple sclerosis disability outcome measures using pooled clinical trial data
- PMID: 31641014
- PMCID: PMC6885577
- DOI: 10.1212/WNL.0000000000008519
Evaluation of multiple sclerosis disability outcome measures using pooled clinical trial data
Erratum in
-
Evaluation of Multiple Sclerosis Disability Outcome Measures Using Pooled Clinical Trial Data.Neurology. 2021 Mar 9;96(10):504-505. doi: 10.1212/WNL.0000000000011677. Epub 2021 Feb 12. Neurology. 2021. PMID: 33579759 Free PMC article. No abstract available.
Abstract
Objective: We report analyses of a pooled database by the Multiple Sclerosis Outcome Assessments Consortium to evaluate 4 proposed components of a multidimensional test battery.
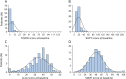
Methods: Standardized data on 12,776 participants, comprising demographics, multiple sclerosis disease characteristics, Expanded Disability Status Scale (EDSS) score, performance measures, and Short Form-36 Physical Component Summary (SF-36 PCS), were pooled from control and treatment arms of 14 clinical trials. Analyses of Timed 25-Foot Walk (T25FW), 9-Hole Peg Test (9HPT), Low Contrast Letter Acuity (LCLA), and Symbol Digit Modalities Test (SDMT) included measurement properties; construct, convergent, and known group validity; and longitudinal performance of the measures individually and when combined into a multidimensional test battery relative to the EDSS and SF-36 to determine sensitivity and clinical meaningfulness.
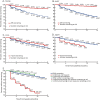
Results: The performance measures had excellent test-retest reliability and showed expected differences between subgroups based on disease duration and EDSS level. Progression rates in detecting time to 3-month confirmed worsening were lower for T25FW and 9HPT compared to EDSS, while progression rates for LCLA and SDMT were similar to EDSS. When the 4 measures were analyzed as a multidimensional measure rather than as individual measures, progression on any one performance measure was more sensitive than the EDSS. Worsening on the performance measures analyzed individually or as a multidimensional test battery was associated with clinically meaningful SF-36 PCS score worsening, supporting clinical meaningfulness of designated performance test score worsening.
Conclusion: These results support the use of the 4 proposed performance measures, individually or combined into a multidimensional test battery as study outcome measures.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
Measuring disability in multiple sclerosis: Walking plus much more.Neurology. 2019 Nov 19;93(21):919-920. doi: 10.1212/WNL.0000000000008515. Epub 2019 Oct 22. Neurology. 2019. PMID: 31641015 No abstract available.
References
-
- Whitaker JN, McFarland HF, Rudge P, Reingold SC. Outcomes assessment in multiple sclerosis clinical trials: a critical analysis. Mult Scler 1995;1:37–47. - PubMed
-
- Rudick R, Antel J, Confavreux C, et al. . Clinical outcomes assessment in multiple sclerosis. Ann Neurol 1996;40:469–479. - PubMed
-
- Rudick R, Antel J, Confavreux C, et al. . Recommendations from the National Multiple Sclerosis Society clinical outcomes assessment task force. Ann Neurol 1997;42:379–382. - PubMed
-
- Schwid SR, Goodman AD, McDermott MP, Bever CF, Cook SD. Quantitative functional measures in MS: what is a reliable change? Neurology 2002;58:1294–1296. - PubMed
-
- Kragt J, Van der Linden F, Nielsen J, Uitdehagg B, Polman C. Clinical impact of 20% worsening on Timed 25-Foot Walk and 9-Hole Peg Test in multiple sclerosis. Mult Scler 2006;12:594–598. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
