Alpha Oscillations in the Human Brain Implement Distractor Suppression Independent of Target Selection
- PMID: 31641052
- PMCID: PMC6891068
- DOI: 10.1523/JNEUROSCI.1954-19.2019
Alpha Oscillations in the Human Brain Implement Distractor Suppression Independent of Target Selection
Abstract
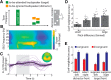
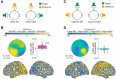
In principle, selective attention is the net result of target selection and distractor suppression. The way in which both mechanisms are implemented neurally has remained contested. Neural oscillatory power in the alpha frequency band (∼10 Hz) has been implicated in the selection of to-be-attended targets, but there is lack of empirical evidence for its involvement in the suppression of to-be-ignored distractors. Here, we use electroencephalography recordings of N = 33 human participants (males and females) to test the preregistered hypothesis that alpha power directly relates to distractor suppression and thus operates independently from target selection. In an auditory spatial pitch discrimination task, we modulated the location (left vs right) of either a target or a distractor tone sequence, while fixing the other in the front. When the distractor was fixed in the front, alpha power relatively decreased contralaterally to the target and increased ipsilaterally. Most importantly, when the target was fixed in the front, alpha lateralization reversed in direction for the suppression of distractors on the left versus right. These data show that target-selection-independent alpha power modulation is involved in distractor suppression. Although both lateralized alpha responses for selection and for suppression proved reliable, they were uncorrelated and distractor-related alpha power emerged from more anterior, frontal cortical regions. Lending functional significance to suppression-related alpha oscillations, alpha lateralization at the individual, single-trial level was predictive of behavioral accuracy. These results fuel a renewed look at neurobiological accounts of selection-independent suppressive filtering in attention.SIGNIFICANCE STATEMENT Although well established models of attention rest on the assumption that irrelevant sensory information is filtered out, the neural implementation of such a filter mechanism is unclear. Using an auditory attention task that decouples target selection from distractor suppression, we demonstrate that two sign-reversed lateralized alpha responses reflect target selection versus distractor suppression. Critically, these alpha responses are reliable, independent of each other, and generated in more anterior, frontal regions for suppression versus selection. Prediction of single-trial task performance from alpha modulation after stimulus onset agrees with the view that alpha modulation bears direct functional relevance as a neural implementation of attention. Results demonstrate that the neurobiological foundation of attention implies a selection-independent alpha oscillatory mechanism to suppress distraction.
Keywords: alpha oscillations; attention; auditory; selection; suppression.
Copyright © 2019 the authors.
Figures



References
-
- Adrian ED. (1944) Brain rhythms. Nature 153:360–362. 10.1038/153360a0 - DOI
-
- Banerjee S, Snyder AC, Molholm S, Foxe JJ (2011) Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J Neurosci 31:9923–9932. 10.1523/JNEUROSCI.4660-10.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
