Induction of metabolic quiescence defines the transitional to follicular B cell switch
- PMID: 31641080
- PMCID: PMC7301641
- DOI: 10.1126/scisignal.aaw5573
Induction of metabolic quiescence defines the transitional to follicular B cell switch
Abstract
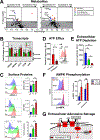
Transitional B cells must actively undergo selection for self-tolerance before maturing into their resting follicular B cell successors. We found that metabolic quiescence was acquired at the follicular B cell stage in both humans and mice. In follicular B cells, the expression of genes involved in ribosome biogenesis, aerobic respiration, and mammalian target of rapamycin complex 1 (mTORC1) signaling was reduced when compared to that in transitional B cells. Functional metabolism studies, profiling of whole-cell metabolites, and analysis of cell surface proteins in human B cells suggested that this transition was also associated with increased extracellular adenosine salvage. Follicular B cells increased the abundance of the cell surface ectonucleotidase CD73, which coincided with adenosine 5'-monophosphate-activated protein kinase (AMPK) activation. Differentiation to the follicular B cell stage in vitro correlated with surface acquisition of CD73 on human transitional B cells and was augmented with the AMPK agonist, AICAR. Last, individuals with gain-of-function PIK3CD (PI3Kδ) mutations and increased pS6 activation exhibited a near absence of circulating follicular B cells. Together, our data suggest that mTORC1 attenuation may be necessary for human follicular B cell development. These data identify a distinct metabolic switch during human B cell development at the transitional to follicular stages, which is characterized by an induction of extracellular adenosine salvage, AMPK activation, and the acquisition of metabolic quiescence.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests:
A.K.S. is a founder and on the scientific advisory board of Honeycomb Biotechnologies, which is developing Seq-Well arrays for commercial use. All other authors declare that they have no competing interests.
Figures




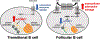
References
-
- Nutt SL, Kee BL, The transcriptional regulation of B cell lineage commitment. Immunity 26, 715–725 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL095791/HL/NHLBI NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- U19 AI110495/AI/NIAID NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01 HL134539/HL/NHLBI NIH HHS/United States
- R01 AI138546/AI/NIAID NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R33 CA202820/CA/NCI NIH HHS/United States
- K08 AI113163/AI/NIAID NIH HHS/United States
- U24 AI118672/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- RM1 HG006193/HG/NHGRI NIH HHS/United States
- T32 HL116275/HL/NHLBI NIH HHS/United States
- U19 AI089992/AI/NIAID NIH HHS/United States
- K08 HL145095/HL/NHLBI NIH HHS/United States
- U54 CA217377/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

