Mitochondrial 3243A > G mutation confers pro-atherogenic and pro-inflammatory properties in MELAS iPS derived endothelial cells
- PMID: 31641105
- PMCID: PMC6805858
- DOI: 10.1038/s41419-019-2036-9
Mitochondrial 3243A > G mutation confers pro-atherogenic and pro-inflammatory properties in MELAS iPS derived endothelial cells
Abstract
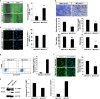
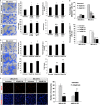
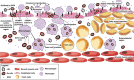
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome is a mitochondrial disorder that is commonly caused by the m.3243A > G mutation in the MT-TL1 gene encoding for mitochondrial tRNA(Leu(UUR)). While clinical studies reported cerebral infarcts, atherosclerotic lesions, and altered vasculature and stroke-like episodes (SLE) in MELAS patients, it remains unclear how this mutation causes the onset and subsequent progression of the disease. Here, we report that in addition to endothelial dysfunction, diseased endothelial cells (ECs) were found to be pro-atherogenic and pro-inflammation due to high levels of ROS and Ox-LDLs, and high basal expressions of VCAM-1, in particular isoform b, respectively. Consistently, more monocytes were found to adhere to MELAS ECs as compared to the isogenic control, suggesting the presence of an atherosclerosis-like pathology in MELAS. Notably, these disease phenotypes in endothelial cells can be effectively reversed by anti-oxidant treatment suggesting that the lowering of ROS is critical for treating patients with MELAS syndrome.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options.Mol Genet Metab. 2015 Sep-Oct;116(1-2):4-12. doi: 10.1016/j.ymgme.2015.06.004. Epub 2015 Jun 15. Mol Genet Metab. 2015. PMID: 26095523 Review.
-
Acute myocardial infarction in a patient with MELAS syndrome: a possible link?Minerva Cardiol Angiol. 2023 Aug;71(4):374-380. doi: 10.23736/S2724-5683.22.06021-5. Epub 2022 Jun 29. Minerva Cardiol Angiol. 2023. PMID: 35767235
-
The MELAS mutation m.3243A>G promotes reactivation of fetal cardiac genes and an epithelial-mesenchymal transition-like program via dysregulation of miRNAs.Biochim Biophys Acta Mol Basis Dis. 2018 Sep;1864(9 Pt B):3022-3037. doi: 10.1016/j.bbadis.2018.06.014. Epub 2018 Jun 19. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29928977
-
The MELAS mutation m.3243A>G alters the expression of mitochondrial tRNA fragments.Biochim Biophys Acta Mol Cell Res. 2019 Sep;1866(9):1433-1449. doi: 10.1016/j.bbamcr.2019.06.004. Epub 2019 Jun 11. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31195049
-
Molecular pathology of MELAS and L-arginine effects.Biochim Biophys Acta. 2012 May;1820(5):608-14. doi: 10.1016/j.bbagen.2011.09.005. Epub 2011 Sep 14. Biochim Biophys Acta. 2012. PMID: 21944974 Review.
Cited by
-
Mitochondrial Chronic Progressive External Ophthalmoplegia.Brain Sci. 2024 Jan 27;14(2):135. doi: 10.3390/brainsci14020135. Brain Sci. 2024. PMID: 38391710 Free PMC article. Review.
-
Mitochondrial diseases: from molecular mechanisms to therapeutic advances.Signal Transduct Target Ther. 2025 Jan 10;10(1):9. doi: 10.1038/s41392-024-02044-3. Signal Transduct Target Ther. 2025. PMID: 39788934 Free PMC article. Review.
-
Multimodal single-cell analysis of nonrandom heteroplasmy distribution in human retinal mitochondrial disease.JCI Insight. 2023 Jul 24;8(14):e165937. doi: 10.1172/jci.insight.165937. JCI Insight. 2023. PMID: 37289546 Free PMC article.
-
Metabolic remodeling in hiPSC-derived myofibers carrying the m.3243A>G mutation.Stem Cell Reports. 2025 Apr 8;20(4):102448. doi: 10.1016/j.stemcr.2025.102448. Epub 2025 Mar 13. Stem Cell Reports. 2025. PMID: 40086445 Free PMC article.
-
The Role of Estrogen in Mitochondrial Disease.Cell Mol Neurobiol. 2025 Jul 11;45(1):68. doi: 10.1007/s10571-025-01592-8. Cell Mol Neurobiol. 2025. PMID: 40643846 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

