Dynamics of ABC Transporter P-glycoprotein in Three Conformational States
- PMID: 31641149
- PMCID: PMC6805939
- DOI: 10.1038/s41598-019-50578-2
Dynamics of ABC Transporter P-glycoprotein in Three Conformational States
Abstract
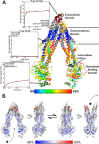
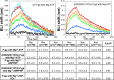
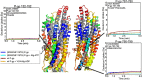
We used hydrogen-deuterium exchange mass spectrometry (HDX-MS) to obtain a comprehensive view of transporter dynamics (85.8% sequence coverage) occurring throughout the multidrug efflux transporter P-glycoprotein (P-gp) in three distinct conformational states: predominantly inward-facing apo P-gp, pre-hydrolytic (E552Q/E1197Q) P-gp bound to Mg+2-ATP, and outward-facing P-gp bound to Mg+2-ADP-VO4-3. Nucleotide affinity was measured with bio-layer interferometry (BLI), which yielded kinetics data that fit a two Mg+2-ATP binding-site model. This model has one high affinity site (3.2 ± 0.3 µM) and one low affinity site (209 ± 25 µM). Comparison of deuterium incorporation profiles revealed asymmetry between the changes undergone at the critical interfaces where nucleotide binding domains (NBDs) contact intracellular helices (ICHs). In the pre-hydrolytic state, both interfaces between ICHs and NBDs decreased exchange to similar extents relative to inward-facing P-gp. In the outward-facing state, the ICH-NBD1 interface showed decreased exchange, while the ICH-NBD2 interface showed less of an effect. The extracellular loops (ECLs) showed reduced deuterium uptake in the pre-hydrolytic state, consistent with an occluded conformation. While in the outward-facing state, increased ECL exchange corresponding to EC domain opening was observed. These findings point toward asymmetry between both NBDs, and they suggest that pre-hydrolytic P-gp occupies an occluded conformation.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

