Mitochondrial Fusion by M1 Promotes Embryoid Body Cardiac Differentiation of Human Pluripotent Stem Cells
- PMID: 31641358
- PMCID: PMC6770295
- DOI: 10.1155/2019/6380135
Mitochondrial Fusion by M1 Promotes Embryoid Body Cardiac Differentiation of Human Pluripotent Stem Cells
Abstract
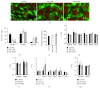
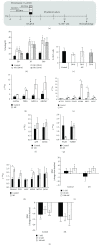
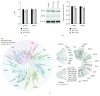
Human induced pluripotent stem cells (iPSCs) can be differentiated in vitro into bona fide cardiomyocytes for disease modelling and personalized medicine. Mitochondrial morphology and metabolism change dramatically as iPSCs differentiate into mesodermal cardiac lineages. Inhibiting mitochondrial fission has been shown to promote cardiac differentiation of iPSCs. However, the effect of hydrazone M1, a small molecule that promotes mitochondrial fusion, on cardiac mesodermal commitment of human iPSCs is unknown. Here, we demonstrate that treatment with M1 promoted mitochondrial fusion in human iPSCs. Treatment of iPSCs with M1 during embryoid body formation significantly increased the percentage of beating embryoid bodies and expression of cardiac-specific genes. The pro-fusion and pro-cardiogenic effects of M1 were not associated with changes in expression of the α and β subunits of adenosine triphosphate (ATP) synthase. Our findings demonstrate for the first time that hydrazone M1 is capable of promoting cardiac differentiation of human iPSCs, highlighting the important role of mitochondrial dynamics in cardiac mesoderm lineage specification and cardiac development. M1 and other mitochondrial fusion promoters emerge as promising molecular targets to generate lineages of the heart from human iPSCs for patient-specific regenerative medicine.
Copyright © 2019 Jarmon G. Lees et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

