P27 Promotes TGF- β-Mediated Pulmonary Fibrosis via Interacting with MTORC2
- PMID: 31641391
- PMCID: PMC6770332
- DOI: 10.1155/2019/7157861
P27 Promotes TGF- β-Mediated Pulmonary Fibrosis via Interacting with MTORC2
Abstract
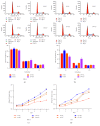
Pulmonary fibrosis (PF), a progressive and life-threatening pulmonary disease, is the main pathological basis of interstitial lung disease (ILD) which includes the idiopathic pulmonary fibrosis (IPF). No effective therapeutic strategy for pulmonary fibrosis has been established. TGF-β signaling has emerged as the vital regulator of PF; however, the detailed molecular mechanisms of TGF-β in PF were uncertain. In the present study, we proved that inhibition of MTORC2 suppresses the expression of P27 in MRC5 and HLF cells. And in bleomycin-induced PF model, the expression of α-SMA and P27 was upregulated. Moreover, TGF-β application increased the level of α-SMA, vimentin, and P27 in MRC5 and HLF cells. Furthermore, P27 overexpression advanced the cell cycle process and promoted the proliferation of MRC5 and HLF cells. Finally, the rescue experiment showed that MTORC2 knockdown reversed P27 overexpression-induced cell cycle acceleration and proliferation. Thus, our results suggest that P27 is involved in TGF-β-mediated PF, which was regulated by MTORC2, providing a novel insight into the development of PF.
Copyright © 2019 Yu-heng Dai et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

