Efficacy and Safety of a Single Dose of Ivermectin, Diethylcarbamazine, and Albendazole for Treatment of Lymphatic Filariasis in Côte d'Ivoire: An Open-label Randomized Controlled Trial
- PMID: 31641754
- PMCID: PMC7583415
- DOI: 10.1093/cid/ciz1050
Efficacy and Safety of a Single Dose of Ivermectin, Diethylcarbamazine, and Albendazole for Treatment of Lymphatic Filariasis in Côte d'Ivoire: An Open-label Randomized Controlled Trial
Abstract
Background: Improved drug regimens are needed to accelerate elimination of lymphatic filariasis in Africa. This study determined whether a single co-administered dose of ivermectin plus diethylcarbamazine plus albendazole [IDA] is noninferior to standard 3 annual doses of ivermectin plus albendazole (IA) used in many LF-endemic areas of Africa.
Methods: Treatment-naive adults with Wuchereria bancrofti microfilaremia in Côte d'Ivoire were randomized to receive a single dose of IDA (n = 43) or 3 annual doses of IA (n = 52) in an open-label, single-blinded trial. The primary endpoint was the proportion of participants who were microfilaria (Mf) negative at 36 months. Secondary endpoints were Mf clearance at 6, 12, and 24 months; inactivation of adult worm nests; and safety.
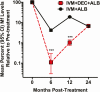
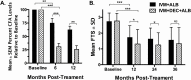
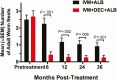
Results: At 36 months posttreatment with IDA, 18/33 (55%; 95% CI, 38-72%) cleared Mf versus 33/42 (79%; 67-91%) with IA (P = .045). At 6 and 12 months IDA was superior to IA in clearing Mf (89% [77-99%] and 71% [56-85%]), respectively, versus 34% (20-48%) and 26% (14-42%) (P < .001). IDA was equivalent to IA at 24 months (61% [45-77%] vs 54% [38-72%]; P = .53). IDA was superior to IA for inactivating adult worms at all time points. Both treatments were well tolerated, and there were no serious adverse events.
Conclusions: A single dose of IDA was superior to 2 doses of IA in reducing the overall Mf burden by 24 months. Reinfection may have contributed to the lack of sustained clearance of Mf with IDA.
Clinical trials registration: NCT02974049.
Keywords: albendazole; diethylcarbamazine; efficacy; ivermectin; lymphatic filariasis.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: An open-label, cluster-randomised trial.PLoS Negl Trop Dis. 2022 Feb 9;16(2):e0010096. doi: 10.1371/journal.pntd.0010096. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35139070 Free PMC article. Clinical Trial.
-
Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Côte d'Ivoire.PLoS Negl Trop Dis. 2019 May 20;13(5):e0007325. doi: 10.1371/journal.pntd.0007325. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31107869 Free PMC article. Clinical Trial.
-
An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India.PLoS Negl Trop Dis. 2021 Feb 16;15(2):e0009069. doi: 10.1371/journal.pntd.0009069. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33591979 Free PMC article. Clinical Trial.
-
Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD003753. doi: 10.1002/14651858.CD003753.pub4. Cochrane Database Syst Rev. 2019. PMID: 30620051 Free PMC article.
-
Model-based analysis of trial data: microfilaria and worm-productivity loss after diethylcarbamazine-albendazole or ivermectin-albendazole combination therapy against Wuchereria bancrofti.Trop Med Int Health. 2006 May;11(5):718-28. doi: 10.1111/j.1365-3156.2006.01606.x. Trop Med Int Health. 2006. PMID: 16640625 Review.
Cited by
-
Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: An open-label, cluster-randomised trial.PLoS Negl Trop Dis. 2022 Feb 9;16(2):e0010096. doi: 10.1371/journal.pntd.0010096. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35139070 Free PMC article. Clinical Trial.
-
Lymphatic filariasis epidemiology in Samoa in 2018: Geographic clustering and higher antigen prevalence in older age groups.PLoS Negl Trop Dis. 2020 Dec 21;14(12):e0008927. doi: 10.1371/journal.pntd.0008927. eCollection 2020 Dec. PLoS Negl Trop Dis. 2020. PMID: 33347456 Free PMC article.
-
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform.PLoS Negl Trop Dis. 2024 Jan 16;18(1):e0011882. doi: 10.1371/journal.pntd.0011882. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38227595 Free PMC article.
-
Antifilarial treatment strategies: a systematic review and network meta-analysis.BMC Infect Dis. 2025 May 16;25(1):712. doi: 10.1186/s12879-025-11105-z. BMC Infect Dis. 2025. PMID: 40380307 Free PMC article. Review.
-
Accelerating Progress Towards the 2030 Neglected Tropical Diseases Targets: How Can Quantitative Modeling Support Programmatic Decisions?Clin Infect Dis. 2024 Apr 25;78(Suppl 2):S83-S92. doi: 10.1093/cid/ciae082. Clin Infect Dis. 2024. PMID: 38662692 Free PMC article.
References
-
- Michael E, Malecela-Lazaro MN, Kazura JW. Epidemiological modelling for monitoring and evaluation of lymphatic filariasis control. Adv Parasitol 2007; 65:191–237. - PubMed
-
- Thomsen EK, Sanuku N, Baea M, et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis 2016; 62:334–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

