Emerging and established modes of cell death during acetaminophen-induced liver injury
- PMID: 31641808
- PMCID: PMC6891214
- DOI: 10.1007/s00204-019-02597-1
Emerging and established modes of cell death during acetaminophen-induced liver injury
Abstract
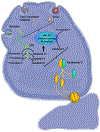
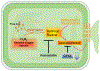
Acetaminophen (APAP)-induced liver injury is an important clinical and toxicological problem. Understanding the mechanisms and modes of cell death are vital for the development of therapeutic interventions. The histological and clinical features of APAP hepatotoxicity including cell and organelle swelling, karyolysis, and extensive cell contents release lead to the characterization of the cell death as oncotic necrosis. However, the more recent identification of detailed signaling mechanisms of mitochondrial dysfunction, the amplification mechanisms of mitochondrial oxidant stress and peroxynitrite formation by a mitogen-activated protein kinase cascade, mechanisms of the mitochondrial permeability transition pore opening and nuclear DNA fragmentation as well as the characterization of the sterile inflammatory response suggested that the mode of cell death is better termed programmed necrosis. Additional features like mitochondrial Bax translocation and cytochrome c release, mobilization of lysosomal iron and the activation of receptor-interacting protein kinases and the inflammasome raised the question whether other emerging modes of cell death such as apoptosis, necroptosis, ferroptosis and pyroptosis could also play a role. The current review summarizes the key mechanisms of APAP-induced liver injury and compares these with key features of the newly described modes of cell death. Based on the preponderance of experimental and clinical evidence, the mode of APAP-induced cell death should be termed programmed necrosis; despite some overlap with other modes of cell death, APAP hepatotoxicity does not fulfill the characteristics of either apoptosis, necroptosis, ferroptosis, pyroptosis or autophagic cell death.
Keywords: Acetaminophen; Apoptosis; Cell death; Drug hepatotoxicity; Ferroptosis; Necrosis.
Conflict of interest statement
Conflict of Interest
H Jaeschke received grant support from McNeil Consumer Health, Inc. All other authors declare no conflict of interest.
Figures

Comment in
-
Acetaminophen induces programmed necrosis.Arch Toxicol. 2019 Dec;93(12):3641-3642. doi: 10.1007/s00204-019-02625-0. Epub 2019 Nov 14. Arch Toxicol. 2019. PMID: 31728590 No abstract available.
-
Role of ferroptosis in acetaminophen-induced hepatotoxicity.Arch Toxicol. 2020 May;94(5):1769-1770. doi: 10.1007/s00204-020-02714-5. Epub 2020 Mar 16. Arch Toxicol. 2020. PMID: 32180037 No abstract available.
-
Response to the opinion letter entitled Role of Ferroptosis in Acetaminophen Hepatotoxicity by Yamada et al.Arch Toxicol. 2020 May;94(5):1771-1772. doi: 10.1007/s00204-020-02723-4. Epub 2020 Apr 2. Arch Toxicol. 2020. PMID: 32240331 No abstract available.
References
-
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA (2001) Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol 60:907–15. - PubMed
-
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H (2006) Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci 94:217–25. - PubMed
-
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H (2008) Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther 324:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

