The pharmacokinetics of 3-fluoroamphetamine following delivery using clinically relevant routes of administration
- PMID: 31642004
- PMCID: PMC6982562
- DOI: 10.1007/s13346-019-00685-4
The pharmacokinetics of 3-fluoroamphetamine following delivery using clinically relevant routes of administration
Abstract
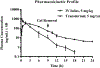
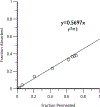
3-Fluoroamphetamine (also called PAL-353) is a synthetic amphetamine analog that has been investigated for cocaine use disorder (CUD), yet no studies have characterized its pharmacokinetics (PK). In the present study, we determined the PK of PAL-353 in male Sprague Dawley rats following intravenous bolus injection (5 mg/kg). Plasma samples were analyzed using a novel bioanalytical method that coupled liquid-liquid extraction and LC-MS/MS. The primary PK parameters determined by WinNonlin were a C0 (ng/mL) of 1412.09 ± 196.12 and a plasma half-life of 2.27 ± 0.67 h. As transdermal delivery may be an optimal approach to delivering PAL-353 for CUD, we assessed its PK profile following application of 50 mg of transdermal gel (10% w/w drug over 5 cm2). The 10% w/w gel resulted in a short lag time, sustained delivery, and a rapid clearance in plasma immediately after removal. The rodent PK data were verified by examining in vitro permeation through human epidermis mounted on Franz diffusion cells. An in vitro-in vivo correlation (IVIVC) analysis was performed using the Phoenix IVIVC toolkit to assess the predictive relationship between rodent and human skin absorption/permeation. The in vitro permeation study revealed a dose-proportional cumulative and steady-state flux with ~ 70% of drug permeated. The fraction absorbed in vivo and fraction permeated in vitro showed a linear relationship. In conclusion, we have characterized the PK profile of PAL-353, demonstrated that it has favorable PK properties for transdermal administration for CUD, and provided preliminary evidence of the capacity of rodent data to predict human skin flux.
Keywords: 3-Fluoroamphetamine; Cocaine use disorder; In vitro-in vivo correlation; Intravenous; Pharmacokinetics; Transdermal.
Conflict of interest statement
Conflicts of Interest
None of the authors has a financial relationship with the sponsor of the research, which was the Georgia Research Alliance. The Georgia Research Alliance is a state government funded agency.
Ajay Banga and Kevin Murnane are cofounders of DD Therapeutics, a for-profit startup company that aims to commercialize drug-delivery technology.
Ajay Banga, Kevin Murnane, and Ying Jiang are co-inventors on a patent pending for transdermal use of phenethylamine monoamine releasers that is owned by Mercer University.
Azizi Ray, Mohammad Shajid Ashraf Junaid, Sonalika Arup Bhattaccharjee, Kayla Kelley, and Bruce Blough have no conflicts of interest to declare.
All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures


References
-
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: The state of the science. Vol. 162, American Journal of Psychiatry; 2005. p. 1432–40. - PubMed
-
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Vol. 102, Addiction; 2007. p. 96–106. - PubMed
-
- Volkow ND, Li T-K. Science and Society: Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004. December;5(12):963–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

