SYNTAX Score and SYNTAX Score II Can Predict the Clinical Outcomes of Patients with Left Main and/or 3-Vessel Disease Undergoing Percutaneous Coronary Intervention in the Contemporary Cobalt-Chromium Everolimus-Eluting Stent Era
- PMID: 31642213
- PMCID: PMC6923234
- DOI: 10.4070/kcj.2019.0097
SYNTAX Score and SYNTAX Score II Can Predict the Clinical Outcomes of Patients with Left Main and/or 3-Vessel Disease Undergoing Percutaneous Coronary Intervention in the Contemporary Cobalt-Chromium Everolimus-Eluting Stent Era
Abstract
Background and objectives: The impact of SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery score (SS) and SS II in patients who receive percutaneous coronary intervention with second-generation everolimus-eluting stents (EES) has not been fully validated.
Methods: The SS, SS II were calculated in 1,248 patients with left main and/or 3-vessel disease treated with EES. Patient-oriented composite endpoint (POCE; all-cause death, any myocardial infarction (MI), any revascularization) and target lesion failure (TLF: cardiac death, target-vessel MI, target lesion revascularization) were analyzed.
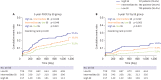
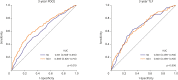
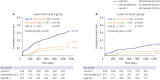
Results: The mean SS was 21.1±9.6. Three-year POCE increased according to the SS group (15.2% vs. 19.9% vs. 27.4% for low (≤22), intermediate (≥23, ≤32), high (≥33) SS groups, p<0.001). By multivariate Cox proportional hazard analysis, SS group was an independent predictor of 3-year POCE (hazard ratio, 1.324; 95% confidence interval, 1.095-1.601; p=0.004). The receiver operating characteristic curves revealed that the SS II was superior to the SS for 3-year POCE prediction (area under the curve [AUC]: 0.611 vs. 0.669 for SS vs. SS II, p=0.019), but not for 3-year TLF (AUC: 0.631 vs. 0.660 for SS vs. SS II, p=0.996). In subgroup analysis, SS II was superior to SS in patients with cardiovascular clinical risk factors, and in those presenting as stable angina.
Conclusions: The usefulness of SS and SS II was still valid in patients with left main and/or 3-vessel disease. SS II was superior to SS for the prediction of patient-oriented outcomes, but not for lesion-oriented outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT00698607, ClinicalTrials.gov Identifier: NCT01605721.
Keywords: Drug-eluting stents; Percutaneous coronary intervention.
Copyright © 2020. The Korean Society of Cardiology.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
References
-
- Kolh P, Windecker S. ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J. 2014;35:3235–3236. - PubMed
-
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;64:e139–e228. - PubMed
-
- Morice MC, Serruys PW, Kappetein AP, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation. 2014;129:2388–2394. - PubMed
-
- Head SJ, Davierwala PM, Serruys PW, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. 2014;35:2821–2830. - PubMed
-
- Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35:1147–1158. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

