Natural History of Treated Subarachnoid Neurocysticercosis
- PMID: 31642423
- PMCID: PMC6947806
- DOI: 10.4269/ajtmh.19-0436
Natural History of Treated Subarachnoid Neurocysticercosis
Abstract
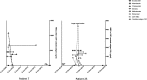
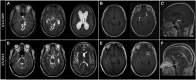
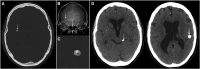
Subarachnoid neurocysticercosis (SUBNCC) is usually caused by an aberrant proliferative form of Taenia solium causing mass effect and arachnoiditis. Thirty of 34 SUBNCC patients were treated with extended cysticidal and anti-inflammatory regimens and followed up a median of 4.2 years posttreatment (range: 15 for ≥ 4 years, 20 ≥ 2 years, 26 > 1 year, and 3 < 1 year). The median ages at the time of first symptom, diagnosis, and enrollment were 29.7, 35.6, and 37.9 years, respectively; 58.8% were male and 82.4% were Hispanic. The median time from immigration to symptoms (minimum incubation) was 10 years and the estimated true incubation period considerably greater. Fifty percent also had other forms of NCC. Common complications were hydrocephalus (56%), shunt placement (41%), infarcts (18%), and symptomatic spinal disease (15%). Thirty patients (88.2%) required prolonged treatment with albendazole (88.2%, median 0.55 year) and/or praziquantel (61.8%; median 0.96 year), corticosteroids (88.2%, median 1.09 years), methotrexate (50%, median 1.37 years), and etanercept (34.2%, median 0.81 year), which led to sustained inactive disease in 29/30 (96.7%) patients. Three were treated successfully for recurrences and one has continuing infection. Normalization of cerebral spinal fluid parameters and cestode antigen levels guided treatment decisions. All 15 patients with undetectable cestode antigen values have sustained inactive disease. There were no deaths and moderate morbidity posttreatment. Corticosteroid-related side effects were common, avascular necrosis of joints being the most serious (8/33, 24.2%). Prolonged cysticidal treatment and effective control of inflammation led to good clinical outcomes and sustained inactive disease which is likely curative.
Figures

Comment in
-
Optimal Treatment for Subarachnoid Neurocysticercosis: Closer, but Not There yet.Am J Trop Med Hyg. 2020 Jan;102(1):1-2. doi: 10.4269/ajtmh.19-0754. Am J Trop Med Hyg. 2020. PMID: 31674300 Free PMC article. No abstract available.
References
-
- Henneberg R, 1912. Die tierischen parasiten des zentralnerven-systems. Lewandowsky M, ed. Handbuch der Neurologie. Berlin, Germany: Verlag Von Julius Springer, 643–712.
-
- Valkounova J, Zdarska Z, Slais J, 1992. Histochemistry of the racemose form of Cysticercus cellulosae. Folia Parasitol (Praha) 39: 207–226. - PubMed
-
- Brown WJ, Voge M, 1982. Neuropathology of Parasitic Infections. Oxford, United Kingdom: Oxford University Press.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

