Assessment of Diagnostic Outcomes of RNA Genetic Testing for Hereditary Cancer
- PMID: 31642931
- PMCID: PMC6820040
- DOI: 10.1001/jamanetworkopen.2019.13900
Assessment of Diagnostic Outcomes of RNA Genetic Testing for Hereditary Cancer
Abstract
Importance: Performing DNA genetic testing (DGT) for hereditary cancer genes is now a well-accepted clinical practice; however, the interpretation of DNA variation remains a challenge for laboratories and clinicians. Adding RNA genetic testing (RGT) enhances DGT by clarifying the clinical actionability of hereditary cancer gene variants, thus improving clinicians' ability to accurately apply strategies for cancer risk reduction and treatment.
Objective: To evaluate whether RGT is associated with improvement in the diagnostic outcome of DGT and in the delivery of personalized cancer risk management for patients with hereditary cancer predisposition.
Design, setting, and participants: Diagnostic study in which patients and/or families with inconclusive variants detected by DGT in genes associated with hereditary breast and ovarian cancer, Lynch syndrome, and hereditary diffuse gastric cancer sent blood samples for RGT from March 2016 to April 2018. Clinicians who ordered genetic testing and received a reclassification report for these variants were surveyed to assess whether RGT-related variant reclassifications changed clinical management of these patients. To quantify the potential number of tested individuals who could benefit from RGT, a cohort of 307 812 patients who underwent DGT for hereditary cancer were separately queried to identify variants predicted to affect splicing. Data analysis was conducted from March 2016 and September 2018.
Main outcomes and measures: Variant reclassification outcomes following RGT, clinical management changes associated with RGT-related variant reclassifications, and the proportion of patients who would likely be affected by a concurrent DGT and RGT multigene panel testing approach.
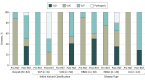
Results: In total, 93 if 909 eligible families (10.2%) submitted samples for RGT. Evidence from RGT clarified the interpretation of 49 of 56 inconclusive cases (88%) studied; 26 (47%) were reclassified as clinically actionable and 23 (41%) were clarified as benign. Variant reclassifications based on RGT results changed clinical management recommendations for 8 of 18 patients (44%) and 14 of 18 families (78%), based on responses from 18 of 45 clinicians (40%) surveyed. A total of 7265 of 307 812 patients who underwent DGT had likely pathogenic variants or variants of uncertain significance potentially affecting splicing, indicating that approximately 1 in 43 individuals could benefit from RGT.
Conclusions and relevance: In this diagnostic study, conducting RNA testing resolved a substantial proportion of variants of uncertain significance in a cohort of individuals previously tested for cancer predisposition by DGT. Performing RGT might change the diagnostic outcome of at least 1 in 43 patients if performed in all individuals undergoing genetic evaluation for hereditary cancer.
Conflict of interest statement
Figures



References
-
- Rubenstein JH, Enns R, Heidelbaugh J, Barkun A; Clinical Guidelines Committee . American Gastroenterological Association Institute guideline on the diagnosis and management of Lynch syndrome. Gastroenterology. 2015;149(3):777-782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

