Perinatal maternal antibiotic exposure augments lung injury in offspring in experimental bronchopulmonary dysplasia
- PMID: 31644311
- PMCID: PMC7132329
- DOI: 10.1152/ajplung.00561.2018
Perinatal maternal antibiotic exposure augments lung injury in offspring in experimental bronchopulmonary dysplasia
Abstract
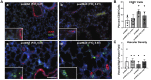
During the newborn period, intestinal commensal bacteria influence pulmonary mucosal immunology via the gut-lung axis. Epidemiological studies have linked perinatal antibiotic exposure in human newborns to an increased risk for bronchopulmonary dysplasia, but whether this effect is mediated by the gut-lung axis is unknown. To explore antibiotic disruption of the newborn gut-lung axis, we studied how perinatal maternal antibiotic exposure influenced lung injury in a hyperoxia-based mouse model of bronchopulmonary dysplasia. We report that disruption of intestinal commensal colonization during the perinatal period promotes a more severe bronchopulmonary dysplasia phenotype characterized by increased mortality and pulmonary fibrosis. Mechanistically, metagenomic shifts were associated with decreased IL-22 expression in bronchoalveolar lavage and were independent of hyperoxia-induced inflammasome activation. Collectively, these results demonstrate a previously unrecognized influence of the gut-lung axis during the development of neonatal lung injury, which could be leveraged to ameliorate the most severe and persistent pulmonary complication of preterm birth.
Keywords: gut-lung axis; interleukin-22; microbiome; mucosal immunology; pulmonary fibrosis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Comment in
-
Maternal antibiotics augment hyperoxia-induced lung injury in neonatal mice.Am J Physiol Lung Cell Mol Physiol. 2020 Feb 1;318(2):L405-L406. doi: 10.1152/ajplung.00442.2019. Epub 2019 Oct 30. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31664856 Free PMC article. No abstract available.
-
The emergence of preclinical studies on the role of the microbiome in lung development and experimental animal models of bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2020 Feb 1;318(2):L402-L404. doi: 10.1152/ajplung.00509.2019. Epub 2020 Jan 22. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31967848 No abstract available.
References
-
- Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 123: 1132–1141, 2009. doi:10.1542/peds.2008-0526. - DOI - PMC - PubMed
-
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, Mohn WW, Turvey SE, Finlay BB; CHILD Study Investigators . Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7: 307ra152, 2015. doi:10.1126/scitranslmed.aab2271. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

