Long-acting injectable cabotegravir for the prevention of HIV infection
- PMID: 31644481
- PMCID: PMC7382946
- DOI: 10.1097/COH.0000000000000597
Long-acting injectable cabotegravir for the prevention of HIV infection
Abstract
Purpose of review: This review highlights the development of long-acting injectable cabotegravir (CAB LA) for HIV preexposure prophylaxis (PrEP), with a focus on phase 2 studies and later development.
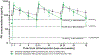
Recent findings: Early studies of CAB LA for HIV prevention offered promising pharmacokinetic data and paved the way for phase 2 studies, which have now been completed. On the basis of phase 2 data, dosing of CAB LA at 8-week intervals consistently delivers target trough concentrations in both men and women. Recent studies have shown no required dose adjustments for hepatic or renal disease and minimal drug--drug interactions. Additionally, injectable PrEP is desired by potential PrEP candidates. Still, gaps in knowledge remain with respect to implementation and delivery, the clinical significance of the pharmacologic tail, and dosing in key populations. Phase 3 trials are underway that are anticipated to inform some of these questions and provide efficacy and safety data to support regulatory submissions for CAB LA as a potential PrEP agent.
Summary: Recent studies have defined an appropriate CAB LA dosing interval and offered insight into its safety profile. Phase 3 studies will provide much-anticipated efficacy data. If efficacious, CAB LA may provide a desirable PrEP option for those who face challenges to daily pill adherence. A more complete understanding of how to best integrate LA PrEP into service delivery models will be critical for success.
Conflict of interest statement
Conflicts of interest
RJL is an Advisory Board member for Gilead Sciences and Merck and receives consultation fees from Roche. MEC receives consulting fees from FHI360.
Figures

References
-
- Hare CB, Coll J, Ruane P, et al. The Phase 3 DISCOVER Study: Daily F/TAF or F/TDF for HIV Pre-exposure Prophylaxis Abstract #0104. CROI, Seattle, WA, 2019.
-
- Corneli A, Perry B, McKenna K, et al. Participants’ Explanations for Nonadherence in the FEM-PrEP Clinical Trial. J Acquir Immune Defic Syndr. 2016;71(4):452–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

