Chemical-Shift Perturbations Reflect Bile Acid Binding to Norovirus Coat Protein: Recognition Comes in Different Flavors
- PMID: 31644826
- PMCID: PMC7186840
- DOI: 10.1002/cbic.201900572
Chemical-Shift Perturbations Reflect Bile Acid Binding to Norovirus Coat Protein: Recognition Comes in Different Flavors
Abstract
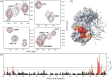
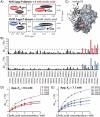
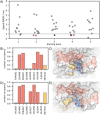
Bile acids have been reported as important cofactors promoting human and murine norovirus (NoV) infections in cell culture. The underlying mechanisms are not resolved. Through the use of chemical shift perturbation (CSP) NMR experiments, we identified a low-affinity bile acid binding site of a human GII.4 NoV strain. Long-timescale MD simulations reveal the formation of a ligand-accessible binding pocket of flexible shape, allowing the formation of stable viral coat protein-bile acid complexes in agreement with experimental CSP data. CSP NMR experiments also show that this mode of bile acid binding has a minor influence on the binding of histo-blood group antigens and vice versa. STD NMR experiments probing the binding of bile acids to virus-like particles of seven different strains suggest that low-affinity bile acid binding is a common feature of human NoV and should therefore be important for understanding the role of bile acids as cofactors in NoV infection.
Keywords: STD NMR spectroscopy; chemical shift perturbation; ensemble docking; long-timescale MD; molecular recognition.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
Figures






References
-
- None
-
- de Graaf M., van Beek J., Koopmans M. P., Nat. Rev. Microbiol. 2016, 14, 421–433; - PubMed
-
- None
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

