Proceedings of the 2019 National Toxicology Program Satellite Symposium
- PMID: 31645210
- PMCID: PMC6911009
- DOI: 10.1177/0192623319876929
Proceedings of the 2019 National Toxicology Program Satellite Symposium
Abstract
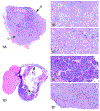
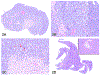
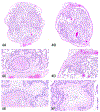
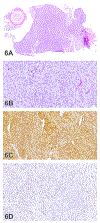
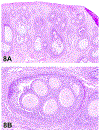
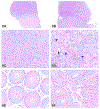
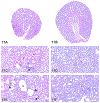
The 2019 annual National Toxicology Program Satellite Symposium, entitled "Pathology Potpourri," was held in Raleigh, North Carolina, at the Society of Toxicologic Pathology's 38th annual meeting. The goal of this symposium was to present and discuss challenging diagnostic pathology and/or nomenclature issues. This article presents summaries of the speakers' talks along with select images that were used by the audience for voting and discussion. Various lesions and topics covered during the symposium included aging mouse lesions from various strains, as well as the following lesions from various rat strains: rete testis sperm granuloma/fibrosis, ovarian cystadenocarcinoma, retro-orbital schwannoma, periductal cholangiofibrosis of the liver and pancreas, pars distalis hypertrophy, chronic progressive nephropathy, and renal tubule regeneration. Other cases included polyovular follicles in young beagle dogs and a fungal blood smear contaminant. One series of cases challenged the audience to consider how immunohistochemistry may improve the diagnosis of some tumors. Interesting retinal lesions from a rhesus macaque emphasized the difficulty in determining the etiology of any particular retinal lesion due to the retina's similar response to vascular injury. Finally, a series of lesions from the International Harmonization of Nomenclature and Diagnostic Criteria Non-Rodent Fish Working Group were presented.
Keywords: INHAND; NTP satellite symposium; aging mouse lesions; ovarian cystadenoma; periductal cholangiofibrosis; polyovular follicles; sperm granuloma.
Conflict of interest statement
Declaration of Conflicting Interests Statement
The author(s) declared no potential, real, or perceived conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

















References
-
- goRENI. https://www.goreni.org/ Accessed July 15, 2019.
-
- Cora M, Travlos G. Bone Marrow—Hypocellularity. National Toxicology Program Nonneoplastic Lesion Atlas 2014; https://ntp.niehs.nih.gov/nnl/hematopoietic/bone_marrow/hypocell/index.htm Accessed July 10, 2019.
-
- Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol 2004;32(1):79–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

