A Dynamic Variation of Pulmonary ACE2 Is Required to Modulate Neutrophilic Inflammation in Response to Pseudomonas aeruginosa Lung Infection in Mice
- PMID: 31645418
- PMCID: PMC7458157
- DOI: 10.4049/jimmunol.1900579
A Dynamic Variation of Pulmonary ACE2 Is Required to Modulate Neutrophilic Inflammation in Response to Pseudomonas aeruginosa Lung Infection in Mice
Abstract
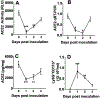
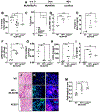
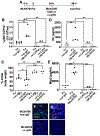
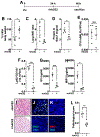
Angiotensin-converting enzyme 2 (ACE2) is a potent negative regulator capable of restraining overactivation of the renin-angiotensin system, which contributes to exuberant inflammation after bacterial infection. However, the mechanism through which ACE2 modulates this inflammatory response is not well understood. Accumulating evidence indicates that infectious insults perturb ACE2 activity, allowing for uncontrolled inflammation. In the current study, we demonstrate that pulmonary ACE2 levels are dynamically varied during bacterial lung infection, and the fluctuation is critical in determining the severity of bacterial pneumonia. Specifically, we found that a pre-existing and persistent deficiency of active ACE2 led to excessive neutrophil accumulation in mouse lungs subjected to bacterial infection, resulting in a hyperinflammatory response and lung damage. In contrast, pre-existing and persistent increased ACE2 activity reduces neutrophil infiltration and compromises host defense, leading to overwhelming bacterial infection. Further, we found that the interruption of pulmonary ACE2 restitution in the model of bacterial lung infection delays the recovery process from neutrophilic lung inflammation. We observed the beneficial effects of recombinant ACE2 when administered to bacterially infected mouse lungs following an initial inflammatory response. In seeking to elucidate the mechanisms involved, we discovered that ACE2 inhibits neutrophil infiltration and lung inflammation by limiting IL-17 signaling by reducing the activity of the STAT3 pathway. The results suggest that the alteration of active ACE2 is not only a consequence of bacterial lung infection but also a critical component of host defense through modulation of the innate immune response to bacterial lung infection by regulating neutrophil influx.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures





References
-
- Bijani B, Mozhdehipanah H, Jahanihashemi H, and Azizi S. 2014. The impact of pneumonia on hospital stay among patients hospitalized for acute stroke. Neurosciences (Riyadh) 19: 118–123. - PubMed
-
- Li H, and Cao B. 2017. Pandemic and Avian Influenza A Viruses in Humans: Epidemiology, Virology, Clinical Characteristics, and Treatment Strategy. Clin Chest Med 38: 59–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL121791/HL/NHLBI NIH HHS/United States
- R01 AI111605/AI/NIAID NIH HHS/United States
- R01 HL120153/HL/NHLBI NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- T32 DK007713/DK/NIDDK NIH HHS/United States
- R35 HL145242/HL/NHLBI NIH HHS/United States
- T32 OD011089/OD/NIH HHS/United States
- I01 BX004294/BX/BLRD VA/United States
- R01 AI130591/AI/NIAID NIH HHS/United States
- U19 AI070412/AI/NIAID NIH HHS/United States
- R01 HL093178/HL/NHLBI NIH HHS/United States
- P30 DK089502/DK/NIDDK NIH HHS/United States
- P50 HL107183/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

