Jensen's force and the statistical mechanics of cortical asynchronous states
- PMID: 31645611
- PMCID: PMC6811577
- DOI: 10.1038/s41598-019-51520-2
Jensen's force and the statistical mechanics of cortical asynchronous states
Abstract
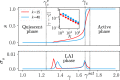
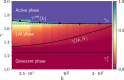
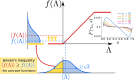
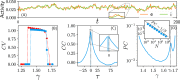
Cortical networks are shaped by the combined action of excitatory and inhibitory interactions. Among other important functions, inhibition solves the problem of the all-or-none type of response that comes about in purely excitatory networks, allowing the network to operate in regimes of moderate or low activity, between quiescent and saturated regimes. Here, we elucidate a noise-induced effect that we call "Jensen's force" -stemming from the combined effect of excitation/inhibition balance and network sparsity- which is responsible for generating a phase of self-sustained low activity in excitation-inhibition networks. The uncovered phase reproduces the main empirically-observed features of cortical networks in the so-called asynchronous state, characterized by low, un-correlated and highly-irregular activity. The parsimonious model analyzed here allows us to resolve a number of long-standing issues, such as proving that activity can be self-sustained even in the complete absence of external stimuli or driving. The simplicity of our approach allows for a deep understanding of asynchronous states and of the phase transitions to other standard phases it exhibits, opening the door to reconcile, asynchronous-state and critical-state hypotheses, putting them within a unified framework. We argue that Jensen's forces are measurable experimentally and might be relevant in contexts beyond neuroscience.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Pastor-Satorras R, Castellano C, Van Mieghem P, Vespignani A. Epidemic processes in complex networks. Rev. Mod. Phys. 2015;87:925. doi: 10.1103/RevModPhys.87.925. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

