Altered dendritic spine function and integration in a mouse model of fragile X syndrome
- PMID: 31645626
- PMCID: PMC6811549
- DOI: 10.1038/s41467-019-11891-6
Altered dendritic spine function and integration in a mouse model of fragile X syndrome
Abstract
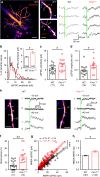
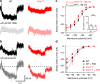
Cellular and circuit hyperexcitability are core features of fragile X syndrome and related autism spectrum disorder models. However, the cellular and synaptic bases of this hyperexcitability have proved elusive. We report in a mouse model of fragile X syndrome, glutamate uncaging onto individual dendritic spines yields stronger single-spine excitation than wild-type, with more silent spines. Furthermore, fewer spines are required to trigger an action potential with near-simultaneous uncaging at multiple spines. This is, in part, from increased dendritic gain due to increased intrinsic excitability, resulting from reduced hyperpolarization-activated currents, and increased NMDA receptor signaling. Using super-resolution microscopy we detect no change in dendritic spine morphology, indicating no structure-function relationship at this age. However, ultrastructural analysis shows a 3-fold increase in multiply-innervated spines, accounting for the increased single-spine glutamate currents. Thus, loss of FMRP causes abnormal synaptogenesis, leading to large numbers of poly-synaptic spines despite normal spine morphology, thus explaining the synaptic perturbations underlying circuit hyperexcitability.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

